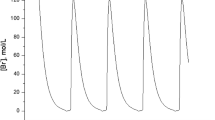
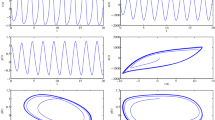
Abstract
This paper presents a numerical study of the reaction A ↔ B in the presence of an intermediate and destabilizing step in its dynamics. After introducing a direct autocatalytic destabilizing process, namely quadratic autocatalysis (\(X + Y \to 2Y\)) and cubic autocatalysis (\(X + 2Y \to 3Y\)), a thermodynamic analysis of the evolution of the reaction in closed and open systems was performed. In addition, the Gibbs free energy, the thermodynamic affinity, and the entropy generation of the overall reaction were evaluated for each of the autocatalytic steps, in order to analyze the behavior of these thermodynamic quantities when the system moved towards equilibrium or towards oscillatory non-equilibrium states.








Similar content being viewed by others
References
Aris, R., Gray, P., Scott, S.K.: Modelling cubic autocatalysis by successive bimolecular steps. Chem. Eng. Sci. 43, 207 (1988)
Borckmans, P., Metens, S.: An excursion in theoretical nonlinear chemistry: from oscillations to turing patterns. In: Borckmans, P., de Kepper, P., Khokhlov, A.R., Métens, S. (eds.) Chemomechanical Instabilities in Responsive Materials. Springer, Netherlands, pp. 57–94 (2009)
Cook, G.B., Gray, P., Knapp, D.G., Scott, S.K.: Bimolecular routes to cubic autocatalysis. J. Phys. Chem. 93, 2749 (1989)
de Groot, S.R., Mazur, P.: Non-equilibrium Thermodynamics. Dover, New York (2011)
Demirel, Y.: Nonequilibrium Thermodynamics: Transport and Rate Processes in Physical, Chemical and Biological Systems. Elsevier, North Holland (2007)
Demirel, Y.: Nonequilibrium thermodynamics modeling of coupled biochemical cycles in living cells. J. Non Newton. Fluid. 165, 953 (2010)
Epstein, I.: Oscillating chemical reactions and nonlinear dynamics. J. Chem. Educ. 66, 195 (1989)
Epstein, I.R., Pojman, J.: An introduction to nonlinear chemical dynamics: oscillations, waves, patterns, and chaos. Oxford University Press, Oxford (1998)
Field, R.J., Koros, E., Noyes, R.M.: Oscillations in chemical systems. II. Thorough analysis of temporal oscillation in the bromate–cerium–malonic acid system. J. Am. Chem. Soc. 94, 8649 (1972)
Garcia-Colín, L.S., Piña, E., de la Selva, S.M.T.: On the thermodynamic basis of the affinity decay rate. J. Chem. Phys. 92, 3545 (1990)
Garfinkle, M.: The thermodynamic natural path in chemical reaction kinetics. Discret. Dyn. Nat. Soc. 4, 145 (2000)
Gray, P., Scott, S.K.: Chemical Oscillations and Instabilities, Nonlinear Chemical Kinetics. Clarendon, Oxford (1990)
Irvin, B.R., Ross, J.: Calculation of the rate of entropy production for a model chemical reaction. J. Chem. Phys. 89, 1064 (1988)
Kay, S.R., Scott, S.K., Tomlin, A.S.: Quadratic autocatalysis in a non-isothermal CSTR. Chem. Eng. Sci. 44, 1129 (1989)
Kondepudi, D.K., Prigogine, I.: From Heat Engines to Dissipative Structures. Wiley, New York (1998)
Lefever, R., Nicolis, G., Borckmans, P.: The Brusselator: it does oscillate all the same. J. Chem. Soc. Faraday Trans. 84, 1013 (1988)
Li, Y., Qian, H., Yi, Y.: Oscillations and multiscale dynamics in a closed chemical reaction system: second law of thermodynamics and temporal complexity. J. Chem. Phys. 129, 154505 (2008)
Martyushev, L.M., Seleznev, V.D.: Maximum entropy production principle in physics, chemistry and biology. Phys. Rep. 426, 1 (2006)
Pacault, A., Hanusse, P., De Kepper, P., Vidal, C., Boissonade, J.: Phenomena in homogeneous chemical systems far from equilibrium. Acc. Chem. Res. 9, 438 (1976)
Prigogine, I., Defay, R.: Chemical Thermodynamics. Longmans, London (1954)
Prigogine, I.: Introduction to Thermodynamics of Irreversible Processes. Interscience, New York (1967)
Rastogi, R.P., Mathur, P.: Open systems in non-equilibrium: complexity, dynamics, modeling and mechanism. J. Sci. Ind. Res. 71, 453 (2012)
Rastogi, R.P.: Introduction to Non-equilibrium Physical Chemistry. Elsevier, Ámsterdam (2007)
Ross, J., García-Colín, L.S.: Thermodynamics of chemical systems far from equilibrium. J. Phys. Chem. 93, 2091 (1989)
Ross, J., Vlad, M.O.: Exact solutions for the entropy production rate of several irreversible processes. J. Phys. Chem. A 109, 10607 (2005)
Sagués, F., Epstein, I.: Nonlinear chemical dynamics. Dalton Trans. 7, 1201 (2003)
Saikhanov, M.B.: On the thermodynamic and kinetic stability of nonequilibrium systems. Russ. J. Phys. Chem. A 80, 1170 (2006)
Scott, S.K.: Oscillations in simple models of chemical systems. Acc. Chem. Res. 20, 186 (1987)
Tyson, J.J.: Classification of instabilities in chemical reaction systems. J. Chem. Phys. 62, 1010 (1975)
Van Rysselberghe, P.: The fundamentals of chemical thermodynamics. Chem. Rev. 16, 37 (1935)
Vellela, M., Qian, H.: Stochastic dynamics and non-equilibrium thermodynamics of a bistable chemical system: the Schlögl model revisited. J. R. Soc. Interface 6, 925 (2009)
Wilhelm, T., Heinrich, R.: Smallest chemical reaction system with Hopf bifurcation. J. Math. Chem. 17, 1 (1995)
Wang, J.: Modern Thermodynamics, pp. 1–18. Springer, Berlin (2012)
Acknowledgments
In memory of Dr. Alfredo Gómez O. (1943–2011), our friend, colleague and professor for many years in the Departamento de Química, Facultad de Ciencias, Universidad Nacional de Colombia Sede Bogotá. Financial support by the DIME-Universidad Nacional de Colombia (grant 201010013024) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Barragán, D. Essentials of kinetics and thermodynamics for understanding chemical oscillations. Found Chem 17, 93–106 (2015). https://doi.org/10.1007/s10698-015-9221-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10698-015-9221-4