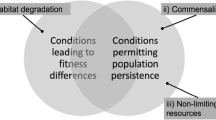
Abstract
The two authors of this paper have diametrically opposed views of the prevalence and strength of adaptation in nature. Hendry believes that adaptation can be seen almost everywhere and that evidence for it is overwhelming and ubiquitous. Gonzalez believes that adaptation is uncommon and that evidence for it is ambiguous at best. Neither author is certifiable to the knowledge of the other, leaving each to wonder where the other has his head buried. Extensive argument has revealed that each author thinks his own view is amply supported by both theory and empirical evidence. Further reflection has revealed that the differences in opinion may start with the different disciplines in which we work: evolutionary ecology for Hendry and community ecology for Gonzalez. In the present paper, we each present devastating evidence supporting our own position and thus refuting that of the other. We then identify the critical differences that led to such opposing views. We close by combining our two perspectives into a common framework based on the adaptive landscape, and thereby suggest means by which to assess the prevalence and strength of adaptation.





Similar content being viewed by others
References
Arnold SJ, Pfrender ME, Jones AG (2001) The adaptive landscape as a bridge between micro- and macroevolution. Genetica 112–113:9–32. doi:10.1023/A:1013373907708
Barton N, Partridge L (2000) Limits to natural selection. Bioessays 22:1075–1084. doi :10.1002/1521-1878(200012)22:12<1075::AID-BIES5>3.0.CO;2-M
Bell G (2001) Neutral macroecology. Science 293:2413–2418. doi:10.1126/science.293.5539.2413
Bell G (2008) Selection: the mechanism of evolution, 2nd edn. Oxford University Press, Oxford, UK
Benkman CW (2003) Divergent selection drives the adaptive radiation of crossbills. Evol Int J Org Evol 57:1176–1181
Berry RJ (1964) The evolution of an island population of the house mouse. Evol Int J Org Evol 18:468–483. doi:10.2307/2406357
Bolnick DI, Nosil P (2007) Natural selection in populations subject to migration load. Evol Int J Org Evol 61:2229–2243. doi:10.1111/j.1558-5646.2007.00179.x
Both C, Bouwhuis S, Lessells CM, Visser ME (2006) Climate change and population declines in a long-distance migratory bird. Nature 441:81–83. doi:10.1038/nature04539
Brandon R (1990) Adaptation and environment. Princeton University Press, Princeton
Burt A (1995) The evolution of fitness. Evol Int J Org Evol 49:1–8. doi:10.2307/2410288
Byars SG, Papst W, Hoffmann AA (2007) Local adaptation and cogradient selection in the alpine plant, Poa hiemata, along a narrow altitudinal gradient. Evol Int J Org Evol 61:2925–2941. doi:10.1111/j.1558-5646.2007.00248.x
Case TJ, Taper ML (2000) Interspecific competition, environmental gradients, gene flow, and the coevolution of species’ borders. Am Nat 155:583–605. doi:10.1086/303351
Caswell H (1976) Community structure: a neutral model analysis. Ecol Monogr 46:327–354. doi:10.2307/1942257
Charlesworth B, Lande R, Slatkin M (1982) A neo-Darwinian commentary on macroevolution. Evol Int J Org Evol 36:474–498. doi:10.2307/2408095
Chase JM, Leibold MA (2003) Ecological niches: linking classical and contemporary approaches. University of Chicago Press, Chicago
Chesson P (2000) Mechanisms of maintenance of species diversity. Annu Rev Ecol Syst 31:343–366. doi:10.1146/annurev.ecolsys.31.1.343
Cox GW (2004) Alien species and evolution. Island Press, Washington
Coyne JA, Orr HA (2004) Speciation. Sinauer Associates, Sunderland
Crespi BJ (2000) The evolution of maladaptation. Heredity 84:623–629. doi:10.1046/j.1365-2540.2000.00746.x
Dieckmann U, Ferrière R (2004) Adaptive dynamics and evolving biodiversity. In: Ferrière R, Dieckmann U, Couvet D (eds) Evolutionary conservation biology. Cambridge University Press, Cambridge, UK, pp 188–224
Dieckmann U, Doebeli M, Metz JAJ, Tautz D (2004) Adaptive speciation. Cambridge University Press, Cambridge
Endler JA (1986) Natural selection in the wild. Princeton University Press, Princeton
Estes S, Arnold SJ (2007) Resolving the paradox of stasis: models with stabilizing selection explain evolutionary divergence on all timescales. Am Nat 169:227–244. doi:10.1086/510633
Facon B, Genton BJ, Shykoff J, Jarne P, Estoup A, David P (2006) A general eco-evolutionary framework for understanding bioinvasions. Trends Ecol Evol 21:130–135. doi:10.1016/j.tree.2005.10.012
Fisher RA (1930) The genetical theory of natural selection. Clarendon Press, Oxford, UK
Frank S (2007) Maladaptation and the paradox of robustness in evolution. PLoS One 10:e1021
Funk DJ, Nosil P, Etges WJ (2006) Ecological divergence exhibits consistently positive associations with reproductive isolation across disparate taxa. Proc Natl Acad Sci USA 103:3209–3213. doi:10.1073/pnas.0508653103
Fussmann GF, Loreau M, Abrams PA (2007) Eco-evolutionary dynamics of communities and ecosystems. Funct Ecol 21:465–477. doi:10.1111/j.1365-2435.2007.01275.x
Gaggiotti OE, Smouse PE (1996) Stochastic migration and maintenance of genetic variation in sink populations. Am Nat 147:919–945. doi:10.1086/285886
Gandon S, Michalakis Y (2002) Local adaptation, evolutionary potential and host parasite coevolution: interactions between migration, mutation, population size and generation time. J Evol Biol 15:451–462. doi:10.1046/j.1420-9101.2002.00402.x
Gandon S, Ebert D, Olivieri I, Michalakis Y (1998) Differential adaptation in spatially heterogeneous environments and host-parasite coevolution. In: Mopper S, Strauss SY (eds) Genetic structure and local adaptation in natural insect populations: effects of ecology, life history ad behavior. Chapman and Hall, New York, pp 325–342
Garant D, Forde SE, Hendry AP (2007) The multifarious effects of dispersal and gene flow on contemporary adaptation. Funct Ecol 21:434–443. doi:10.1111/j.1365-2435.2006.01228.x
García-Ramos G, Kirkpatrick M (1997) Genetic models of adaptation and gene flow in peripheral populations. Evol Int J Org Evol 51:21–28. doi:10.2307/2410956
García-Ramos G, Rodríguez D (2002) Evolutionary speed of species invasions. Evol Int J Org Evol 56:661–668. doi:10.1554/0014-3820(2002)056[0661:ESOSI]2.0.CO;2
Gienapp P, Teplitsky C, Alho JS, Mills JA, Merilä J (2008) Climate change and evolution: disentangling environmental and genetic responses. Mol Ecol 17:167–178
Gillespie JH (1991) The causes of molecular evolution. Oxford Univ Press, Oxford
Gonzalez A, Holt RD (2002) The inflationary effects of environmental fluctuations in source–sink systems. Proc Natl Acad Sci USA 99:14872–14877. doi:10.1073/pnas.232589299
Gonzalez A, Lawton JH, Gilbert FS, Blackburn TM, Evans-Freke I (1998) Metapopulation dynamics, abundance and distribution in a microecosystem. Science 281:2045–2047. doi:10.1126/science.281.5385.2045
Gould SJ (2002) The structure of evolutionary theory. The Belknap Press of Harvard University Press, Cambridge
Gould SJ, Lewontin RC (1979) The spandrels of San Marco and the Panglossian paradigm: a critique of the adaptationist programme. Proc R Soc Lond B Biol Sci 205:581–598. doi:10.1098/rspb.1979.0086
Grant PR, Grant BR (2002) Unpredictable evolution in a 30-year study of Darwin’s finches. Science 296:707–711. doi:10.1126/science.1070315
Gyllenberg M, Parvinen K (2001) Necessary and sufficient conditions for evolutionary suicide. Bull Math Biol 63:981–993. doi:10.1006/bulm.2001.0253
Hairston NG Jr, Ellner SP, Geber MA, Yoshida T, Fox JA (2005) Rapid evolution and the convergence of ecological and evolutionary time. Ecol Lett 8:1114–1127. doi:10.1111/j.1461-0248.2005.00812.x
Haldane JBS (1930) A mathematical theory of natural and artificial selection. VI. Isolation. Proc Camb Philos Soc 26:220–230
Haldane JBS (1956) The relation between density regulation and natural selection. Proc R Soc Lond B Biol Sci 145:306–308. doi:10.1098/rspb.1956.0039
Hansen TF (1997) Stabilizing selection and the comparative analysis of adaptation. Evol Int J Org Evol 51:1341–1351. doi:10.2307/2411186
Hansen TF, Carter AJR, Pélabon C (2006) On adaptive accuracy and precision in natural populations. Am Nat 168:168–181. doi:10.1086/505768
Hanski I, Saccheri I (2006) Molecular-level variation affects population growth in a butterfly metapopulation. PLoS Biol 4:e129. doi:10.1371/journal.pbio.0040129
Hendry AP (2005) The power of natural selection. Nature 433:694–695. doi:10.1038/433694a
Hendry AP, Kinnison MT (1999) The pace of modern life: measuring rates of contemporary microevolution. Evol Int J Org Evol 53:1637–1653. doi:10.2307/2640428
Hendry AP, Taylor EB (2004) How much of the variance in adaptive divergence can be explained by gene flow? An evaluation using lake-stream stickleback pairs. Evol Int J Org Evol 58:2319–2331
Hendry AP, Nosil P, Rieseberg LH (2007) The speed of ecological speciation. Funct Ecol 21:455–464. doi:10.1111/j.1365-2435.2007.01240.x
Hendry AP, Farrugia TJ, Kinnison MT (2008) Human influences on rates of phenotypic change in wild animal populations. Mol Ecol 17:20–29
Hereford J, Hansen TF, Houle D (2004) Comparing strengths of directional selection: how strong is strong? Evol Int J Org Evol 58:2133–2143
Hersch EI, Phillips PC (2004) Power and potential bias in field studies of natural selection. Evol Int J Org Evol 58:479–485
Hoeksema JD, Forde SE (2008) A meta-analysis of factors affecting local adaptation between interaction species. Am Nat 171:275–290. doi:10.1086/527496
Holt RD (1985) Population dynamics in two-patch environments: some anomalous consequences of an optimal habitat distribution. Theor Popul Biol 28:181–208. doi:10.1016/0040-5809(85)90027-9
Holt RD, Gomulkiewicz R (1997) How does immigration influence local adaptation? A reexamination of a familiar paradigm. Am Nat 149:563–572. doi:10.1086/286005
Holt RD, Gomulkiewicz R (2004) Conservation implications of niche conservatism and evolution in heterogeneous environments. In: Ferrière R, Dieckmann U, Couvet D (eds) Evolutionary conservation biology. Cambridge University Press, Cambridge, UK, pp 244–264
Hubbell SP (2001) The unified neutral theory of biodiversity and biogeography. Princeton University Press, Princeton, NJ
Hunt G (2007) The relative importance of directional change, random walks, and stasis in the evolution of fossil lineages. Proc Natl Acad Sci USA 104:18404–18408. doi:10.1073/pnas.0704088104
Hunt G, Bell MA, Travis MP (2008) Evolution toward a new adaptive optimum: phenotypic evolution in a fossil stickleback lineage. Evol Int J Org Evol . doi:10.1111/j.1558-5646.2007.00310.x
Jain SK, Bradshaw AD (1966) Evolutionary divergence among adjacent plant populations. I. The evidence and its theoretical analysis. Heredity 21:407–441. doi:10.1038/hdy.1966.42
Johnston RF, Selander RK (1964) House sparrows: rapid evolution of races in North America. Science 144:548–550. doi:10.1126/science.144.3618.548
Kawecki TJ, Ebert D (2004) Conceptual issues in local adaptation. Ecol Lett 7:1225–1241. doi:10.1111/j.1461-0248.2004.00684.x
Kimura M (1983) The neutral theory of molecular evolution. Cambridge University Press, Cambridge, UK
Kingsolver JG, Pfennig DW (2007) Patterns and power of phenotypic selection in nature. Bioscience 57:561–572. doi:10.1641/B570706
Kingsolver JG, Hoekstra HE, Hoekstra JM, Berrigan D, Vignieri SN, Hill CE et al (2001) The strength of phenotypic selection in natural populations. Am Nat 157:245–261. doi:10.1086/319193
Kinnison MT, Hairston NG Jr (2007) Eco-evolutionary conservation biology: contemporary evolution and the dynamics of persistence. Funct Ecol 21:444–454. doi:10.1111/j.1365-2435.2007.01278.x
Kinnison MT, Hendry AP (2001) The pace of modern life II: from rates of contemporary microevolution to pattern and process. Genetica 112–113:145–164. doi:10.1023/A:1013375419520
Kinnison MT, Unwin MJ, Quinn TP (2008) Eco-evolutionary versus habitat contributions to invasion in salmon: experimental evaluation in the wild. Mol Ecol 17:405–414
Kirkpatrick M, Barton NH (1997) Evolution of a species’ range. Am Nat 150:1–23. doi:10.1086/286054
Knapcyzk FN, Conner JK (2007) Estimates of the average strength of natural selection are not inflated by sampling error or publication bias. Am Nat 170:501–508. doi:10.1086/521239
Lack D (1947) Darwin’s finches. Cambridge University Press, Cambridge, UK
Lande R, Shannon S (1996) The role of genetic variation in adaptation and population persistence in a changing environment. Evol Int J Org Evol 50:434–437. doi:10.2307/2410812
Leigh EG Jr (2007) Neutral theory: a historical perspective. J Evol Biol 20:2075–2091. doi:10.1111/j.1420-9101.2007.01410.x
Lenormand T (2002) Gene flow and the limits to natural selection. Trends Ecol Evol 17:183–189. doi:10.1016/S0169-5347(02)02497-7
Lenski RE, Travasino M (1994) Dynamics of adaptation and diversification: a 10,000-generation experiment with bacterial populations. Proc Natl Acad Sci USA 91:6808–6814. doi:10.1073/pnas.91.15.6808
Liu H, Stiling P (2006) Testing the enemy release hypothesis: a review and meta-analysis. Biol Inv 8:1535–1545. doi:10.1007/s10530-005-5845-y
Matsuda H, Abrams PA (1994) Timid consumers: self-extinction due to adaptive change in foraging and anti-predator effort. Theor Popul Biol 45:76–91. doi:10.1006/tpbi.1994.1004
Matthews DP, Gonzalez A (2007) The inflationary effects of environmental fluctuations ensure the persistence of sink metapopulations. Ecology 88:2848–2856. doi:10.1890/06-1107.1
Moore J-S, Gow JL, Taylor EB, Hendry AP (2007) Quantifying the constraining influence of gene flow on adaptive divergence in the lake-stream threespine stickleback system. Evol Int J Org Evol 61:2015–2026. doi:10.1111/j.1558-5646.2007.00168.x
Mouquet N, Loreau M (2002) Coexistence in metacommunities: the regional similarity hypothesis. Am Nat 159:420–426. doi:10.1086/338996
Nagy ES (1997) Selection for native characters in hybrids between two locally adapted plant subspecies. Evol Int J Org Evol 51:1469–1480. doi:10.2307/2411199
Nesse RM (2005) Maladaptation and natural selection. Q Rev Biol 80:62–70. doi:10.1086/431026
Nuismer SL, Gandon S (2008) Moving beyond common-garden and transplant designs: insight into the causes of local adaptation in species interactions. Am Nat 171:658–668. doi:10.1086/587077
O’Neil P (1999) Selection on flowering time: an adaptive fitness surface for nonexistent character combinations. Ecology 80:806–820
Parker JD, Burkepile DE, Hay ME (2006) Opposing effects of native and exotic herbivores on plant invasions. Science 311:1459–1461. doi:10.1126/science.1121407
Pease CPR, Lande R, Bull JJ (1989) A model of population growth, dispersal, and evolution in a changing environment. Ecology 70:1644–1657. doi:10.2307/1938100
Pelletier F, Clutton-Brock T, Pemberton J, Tuljapurkar S, Coulson T (2007) The evolutionary demography of ecological change: linking trait variation and population growth. Science 315:1571–1574. doi:10.1126/science.1139024
Phillips PA, Arnold SJ (1989) Visualizing multivariate selection. Evol Int J Org Evol 43:1209–1220. doi:10.2307/2409357
Phillips BL, Brown GP, Webb JK, Shine R (2006) Invasion and the evolution of speed in toads. Nature 439:803. doi:10.1038/439803a
Reznick DN, Ghalambor CK (2001) The population ecology of contemporary adaptations: what empirical studies reveal about the conditions that promote adaptive evolution. Genetica 112–113:183–198. doi:10.1023/A:1013352109042
Reznick DN, Shaw FH, Rodd FH, Shaw RG (1997) Evaluation of the rate of evolution in natural populations of guppies (Poecilia reticulata). Science 275:1934–1937. doi:10.1126/science.275.5308.1934
Ricciardi A, Ward JM (2006) Comment on “Opposing effects of native and exotic herbivores on plant invasions”. Science 313:298a
Riechert SE (1993) Investigation of potential gene flow limitation of behavioral adaptation in an aridlands spider. Behav Ecol Sociobiol 32:355–363
Rose MR, Lauder GV (1996) Adaptation. Academic Press, New York
Rosenzweig ML (1973) Evolution of the predator isocline. Evol Int J Org Evol 27:84–94. doi:10.2307/2407121
Roy M, Holt RD, Barfield M (2005) Temporal autocorrelation can enhance the persistence and abundance of metapopulations comprised of coupled sinks. Am Nat 166:246–261. doi:10.1086/431286
Rundle HD, Nosil P (2005) Ecological speciation. Ecol Lett 8:336–352. doi:10.1111/j.1461-0248.2004.00715.x
Saccheri I, Hanski I (2006) Natural selection and population dynamics. Trends Ecol Evol 21:341–347. doi:10.1016/j.tree.2006.03.018
Sax DF, Brown JH (2000) The paradox of invasion. Glob Ecol Biogeogr 9:363–371. doi:10.1046/j.1365-2699.2000.00217.x
Schluter D (2000) The ecology of adaptive radiation. Oxford University Press, Oxford, UK
Schluter D, Grant PR (1984) Determinants of morphological patterns in communities of Darwin’s finches. Am Nat 123:175–196. doi:10.1086/284196
Sheets HD, Mitchell CE (2001) Why the null matters: statistical tests, random walks and evolution. Genetica 112–113:105–125. doi:10.1023/A:1013308409951
Simpson GG (1944) Tempo and mode in evolution. Columbia University Press, New York
Simpson GG (1953) The major features of evolution. Columbia University Press, New York
Slatkin M, Maynard Smith J (1979) Models of coevolution. Q Rev Biol 54:233–266. doi:10.1086/411294
Stearns SC, Sage RD (1980) Maladaptation in a marginal population of the mosquito fish, Gambusia affinis. Evol Int J Org Evol 34:65–75. doi:10.2307/2408315
Svensson E, Sinervo B (2000) Experimental excursions on adaptive landscapes: density-dependent selection on egg size. Evol Int J Org Evol 54:1396–1403
Thompson JN, Nuismer SL, Gomulkiewicz R (2002) Coevolution and maladaptation. Integr Comp Biol 42:381–387. doi:10.1093/icb/42.2.381
Urban MC (2006) Maladaptation and mass effects in a metacommunity: consequences for species coexistence. Am Nat 168:28–40. doi:10.1086/505159
Vellend M, Geber MA (2005) Connections between species diversity and genetic diversity. Ecol Lett 8:767–781. doi:10.1111/j.1461-0248.2005.00775.x
Virgl JA, Messier F (2000) Assessment of source–sink theory for predicting demographic rates among habitats that exhibit temporal changes in quality. Can J Zool 78:1483–1493. doi:10.1139/cjz-78-8-1483
Webb C (2003) A complete classification of Darwinian extinction in ecological interactions. Am Nat 161:181–205. doi:10.1086/345858
Williams GC (1966) Adaptation and natural selection. Princeton University Press, Princeton
Williamson M, Fitter A (1996) The varying success of invaders. Ecology 77:1661–1666. doi:10.2307/2265769
Yoshida T, Jones LE, Ellner SP, Fussmann GF, Hairston NG Jr (2003) Rapid evolution drives ecological dynamics in a predator-prey system. Nature 424:303–306. doi:10.1038/nature01767
Acknowledgements
The motivation for this paper was a multi-day argument between Hendry and Gonzalez whilst teaching in a McGill University field course at the Gault Nature Reserve in Québec, Canada. Our fellow teachers, Irene Gregory-Eaves and Gregor Fussmann, happily added fuel to the fire. Additional helpful comments were provided by Graham Bell, Bernie Crespi, Michel Loreau, Joe Hereford, Gene Hunt, Dolph Schluter, and members of the Hendry lab.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hendry, A.P., Gonzalez, A. Whither adaptation?. Biol Philos 23, 673–699 (2008). https://doi.org/10.1007/s10539-008-9126-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10539-008-9126-x