-
PDF
- Split View
-
Views
-
Cite
Cite
Marianela Candolfi, G. Elizabeth Pluhar, Kurt Kroeger, Mariana Puntel, James Curtin, Carlos Barcia, A.K.M. Ghulam Muhammad, Weidong Xiong, Chunyan Liu, Sonali Mondkar, William Kuoy, Terry Kang, Elizabeth A. McNeil, Andrew B. Freese, John R. Ohlfest, Peter Moore, Donna Palmer, Phillip Ng, John D. Young, Pedro R. Lowenstein, Maria G. Castro, Optimization of adenoviral vector-mediated transgene expression in the canine brain in vivo, and in canine glioma cells in vitro, Neuro-Oncology, Volume 9, Issue 3, July 2007, Pages 245–258, https://doi.org/10.1215/15228517-2007-012
Close - Share Icon Share
Abstract
Expression of the immune-stimulatory molecule Fms-like tyrosine kinase 3 ligand (Flt3L) and the conditional cytotoxic enzyme herpes simplex virus type 1 thymidine kinase (HSV1-TK) provides long-term immune-mediated survival of large glioblastoma multiforme (GBM) models in rodents. A limitation for predictive testing of novel antiglioma therapies has been the lack of a glioma model in a large animal. Dogs bearing spontaneous GBM may constitute an attractive large-animal model for GBM, which so far has remained underappreciated. In preparation for a clinical trial in dogs bearing spontaneous GBMs, we tested and optimized adenovirus-mediated transgene expression with negligible toxicity in the dog brain in vivo and in canine J3T glioma cells. Expression of the marker gene β-galactosidase (β-Gal) was higher when driven by the murine (m) than the human (h) cytomegalovirus (CMV) promoter in the dog brain in vivo, without enhanced inflammation. In the canine brain, β-Gal was expressed mostly in astrocytes. β-Gal activity in J3T cells was also higher with the mCMV than the hCMV promoter driving tetracycline-dependent (TetON) transgene expression within high-capacity adenovirus vectors (HC-Ads). Dog glioma cells were efficiently transduced by HC-Ads expressing mCMV-driven HSV1-TK, which induced 90% reduction in cell viability in the presence of ganciclovir. J3T cells were also effectively transduced with HC-Ads expressing Flt3L under the control of the regulatable TetON promoter system, and as predicted, Flt3L release was stringently inducer dependent. HC-Ads encoding therapeutic transgenes under the control of regulatory sequences driven by the mCMV promoter are excellent vectors for the treatment of spontaneous GBM in dogs, which constitute an ideal preclinical animal model.
Glioblastoma multiforme (GBM) is an aggressive and locally invasive primary brain tumor. Every year, approximately 17,000 Americans are diagnosed with GBM and roughly 13,000 succumb to this disease (see the Web sites of the Mayo Clinic, http://www.mayoclinic.com; and the Central Brain Tumor Registry of the United States, http://www.cbtrus.org). Despite advances in surgery, radiation therapy, and chemotherapy, there is still no cure for GBM and the prognosis remains bleak, the average survival being 6-12 months after diagnosis.1-6 Considering the poor prognosis of patients with high-grade gliomas and the local invasive nature of GBM, gene-therapeutic approaches are very attractive. Although gene-therapy approaches are being investigated in many rodent preclinical models and in clinical trials,6-13 the lack of a large-animal model of GBM makes it difficult to predict the clinical outcome of these experimental therapies.
Most preclinical studies for novel therapeutics for GBM are conducted in rodent models,9,14-17 which have proven very useful both to elucidate mechanisms of GBM progression and to test novel therapeutic strategies. However, it is desirable to develop novel animal models that may better predict the therapeutic outcome of novel therapies to be applied in clinical trials. Dogs bearing spontaneous GBM may constitute an attractive large-animal model for this disease.
Dogs live with people; therefore, the canine genome is exposed to the same environmental influences as the human genome, influences that have been proposed to underlie pathophysiological similarities shared by many human and canine cancers.18 The similarities in terms of disease progression, prognosis, and neuropathological characteristics between canine and human GBM,19-23 combined with the sophisticated genomic resources now available for dogs,24 make GBM a valuable model for preclinical research. GBM is a relatively common tumor in dogs, accounting for 12% of brain tumors,19 especially in brachycephalic breeds of dogs, such as Boston terriers and Boxers, which are predisposed to develop GBM20-22 with MRI and histological features similar to human gliobastomas.23 Dogs have already proven to be excellent models for human Duchenne muscular dystrophy,25,26 hemophilia,27 and lysosomal storage diseases.28,29
High levels of transgene expression are beneficial for gene-therapy applications on several accounts; for example, it enables lowering the vector dose needed to achieve efficacy, thereby diminishing any putative adverse side effects produced by tissue damage or inflammatory or immune responses elicited when high loads of viral vectors are used, and this further ensures stable and high levels of transgene expression. We previously showed that the murine cytomegalovirus (mCMV) promoter exerts higher transgene expression than the human CMV (hCMV) promoter in vivo in rat brain30,31 and in vitro in rodent and human glioma cells.32 In the present work, we compared the strength of the mCMV promoter and the hCMV promoter to drive transgene expression in vivo in dog brain and in vitro in J3T dog glioma cells.
Preexisting immunity could impair transgene expression in the brain of outbred dogs and may also trigger immune or inflammatory processes that could lead to undesired neurotoxicity.33-36 Moreover, most human patients undergoing clinical trials will have preexisting systemic antiadenovirus immunity. Around 60% of the human population exhibits serum antiadenovirus-neutralizing antibodies.37 Thus, in the present report, we determined the prevalence of antiadenovirus immunity in outbred dogs. We studied the effectiveness of high-capacity adenoviral vectors (HC-Ads) to transduce dog glioma cells in vitro, as a prelude to using these vectors to implement a clinical trial for GBM in dogs. These vectors are devoid of all viral encoding genes, exhibiting very low immunogenicity and persistent transgene expression, even in the presence of an anti-Ad immune response.31,35,36,38 We compared the transduction efficiency of regulated HC-Ads encoding the tetracycline-dependent (TetON) system under the control of the mCMV promoter and the hCMV promoter. We also tested the ability of two HC-Ads expressing herpes simplex virus type 1 thymidine kinase (HSV1-TK) or Fms-like tyrosine kinase 3 ligand (Flt3L) to transduce dog glioma cells. We found that the mCMV promoter exerted higher transgene expression levels than the hCMV promoter in vivo and in vitro in the context of both first-generation and HC-Ads. HC-Ads expressing HSV1-TK exerted cytotoxic effects in the presence of ganciclovir, and HC-Ad-TetON-Flt3L expressed and secreted high levels of the cytokine, which was tightly regulated by the inducer doxycycline. In summary, our results support the use of HC-Ads for the treatment of spontaneous GBM in dogs, which constitute an ideal large-animal model for preclinical testing of novel anti-GBM therapies.
Materials and Methods
Adenoviruses
Two vector platforms were used for this study: first-generation replication-defective recombinant adenovirus type 5 vectors (Ads) and novel “gutless” HC-Ads. The first-generation vectors express β-galactosidase (β-Gal) under the transcriptional control of either the human (Ad-hCMV-β-Gal) or murine (Ad-mCMV-β-Gal) CMV intermediate early promoter embedded within the E1 region.39 The high-capacity vectors express their transgenes under the control hCMV or mCMV promoters encoded within a vector genome completely devoid of all adenoviral encoding sequences.40 HC-Ads contain the regulatable expression system TetON, which enables transgene expression to be turned on or off, respectively, in the presence or absence of doxycycline (DOX).31
Development of mCMV- and hCMV-Driven Regulatable TetON Switch
The [mCMV-rtTA2SM2-pIRES-tTSKid-pA] and [hCMV-rtTA2SM2-IRES-tTSkid-pA]-kanamycin regulatable TetON switches were generated as described else-where.31,32,41
Engineering of HSV1-TK and Flt3L Encoding Cassettes
The high-capacity shuttle plasmids pSTK120-mCMV-TK-polyA and pBlueScript II KS (+)[TRE-hsFlt3L-polyA]-[mCMV-rtTA2SM2-pIRES-tTSKid-pA]-kana were generated as described by us previously.32
Scale-Up of First-Generation and HC-Ads
First-generation vectors were scaled up in 293 cells as described by us previously.39 High-capacity vectors were rescued and scaled up in Cre recombinase-expressing 293 cell line by using the helper virus (HV) AdNG163 as previously described.40 First-generation vectors were titered in triplicate by endpoint dilution, cytopathic effect assay.39 The titers determined were as follows: Ad-mCMV-β-Gal (RAd36)30 was 8.19 × 1010 plaque-forming units (PFU)/ml and Ad-hCMV-β-Gal (RAd35)35,36,42,43 was 1.64 × 1011 PFU/ml. The titers of HC-Ads, both total viral particles (VP/ml)39 and contaminating HV (PFU/ml),31,39 were as follows: HC-Ad-mCMV-TetON-β-Gal was 1.2 × 1013 VP/ml and 1 × 107 PFU/ml; HC-Ad-hCMV-TetON-β-Gal was 4.8 × 1012 VP/ml and 1 × 106 PFU/ml; HC-Ad-mCMV-TK was 4.6 × 1012 VP/ml and 1 × 105 PFU/ml; and HC-Ad-mCMV-TetON-Flt3L was 7.0 × 1012 VP/ml and 1 × 105 PFU/ml. All viral preparations were free from replication-competent adenovirus and lipopolysaccharide contamination.39
Dogs
Four healthy adult beagles were purchased by University of Minnesota Research Animal Resources from a licensed vendor and kept in a pathogen-free and well-maintained canine housing facility at the Veterinary Medical Center, College of Veterinary Medicine, University of Minnesota. These experiments were conducted according to an approved Institutional Animal Care and Use Committee protocol, and every effort was made to minimize pain and discomfort. The housing area was maintained at 12 h light and 12 h dark at 22° ± 2°C throughout the study. Every dog was fed ad libitum 220 g of standard-maintenance diet daily. Blood from these dogs was obtained on the day of surgery, 24 h after surgery, and on the day of euthanasia. Rectal body temperature was monitored daily.
Anesthesia and Surgery or Gene Delivery
Intracranial gene delivery was performed as we previously described elsewhere.44 One dog per experiment was injected with either adenovirus-expressing β-Gal under the control of the human promoter (Ad-hCMV-β-Gal) or the murine CMV promoter (Ad-mCMV-β-Gal), both of which were diluted with sterile saline to 8 × 108 PFU in a final volume of 50 μl. Five bilateral injections were given to each dog, with the injections spaced 1 cm apart at a depth of 2 cm (from the cortical surface) in 5 μl/site (8 × 107 PFU/injection site) over 5 min.
Clinical Assessment and Euthanasia of Dogs
Body temperature was taken daily with a rectal thermometer. Blood and cerebrospinal fluid (CSF) from the cisternae magna were collected immediately before surgery and three days afterward. Complete blood count, serum chemistry, urinalysis, and CSF analysis were performed by clinical pathology in the Veterinary Medical Center. Seven days after surgery, the dogs were humanely euthanized by i.v. administration of sodium pentobarbital (200 mg/kg). After euthanasia, all dogs were perfused as previously described.44
Antiadenovirus-Neutralizing Antibody Assay
Serum samples were obtained from 17 healthy outbred dogs recruited at the Veterinary Medical Center, College of Veterinary Medicine, University of Minnesota, and the Department of Comparative Medicine at Cedars-Sinai Medical Center, Los Angeles. Neutralizing antiadenovirus titers were determined as previously described by us.15
Cell Cultures
The dog GBM cell line J3T45 was obtained from Dr. Michael Berens (Translation Genomics Research Institute, Phoenix, AZ, USA). Cultures were maintained in Dulbecco's modified Eagle's medium supplemented with 10% (vol/vol) fetal calf serum, 100 U/ml penicillin, 100 U/ml streptomycin, 2 mM l-glutamine, and 2 mM nonessential amino acids. J3T glioma cells were infected with first-generation (Ads) or high-capacity adenoviral vectors (HC-Ads). Cells were infected with Ads expressing β-Gal under the control of the mCMV or hCMV constitutive promoters. Cells were also infected with HC-AdmTetON-β-Gal and HC-Ad-hTetON-β-Gal31 encoding β-Gal under the control of the TetON system driven by the mCMV promoter and the hCMV promoter, respectively. We also tested an HC-Ad expressing HSV1-TK under the control of the mCMV promoter (HC-Ad-TK) and an HC-Ad expressing human Flt3L under the control of a TetON system driven by the mCMV promoter (HC-Ad-TetON-Flt3L). When cells were infected with regulatable HC-Ads, they were incubated for 72 h with the vector in the presence of 1 μg/ml DOX, which activates the transactivator to initiate transgene expression after binding to the tetracycline-responsive element (TRE) promoter.41 After 72 h, cells were processed for β-Gal enzymatic activity assay or immunocytochemistry to determine viability, and the supernatant was collected for Flt3L ELISA.
β-Galactosidase Activity
J3T dog glioma cells (5 × 104/well) were infected with 30 PFU/cell of a first-generation Ad-hCMV-β-Gal or Ad-mCMV-β-Gal for 72 h. Cells were also infected with 30 blue-forming units of HC-Ad-mTetON-β-Gal or HC-Ad-hTetON-β-Gal and incubated for 72 h either with or without 1 μg/ml DOX. β-Gal activity was measured as described previously.31,32
Detection of HSV1-TK Cytotoxicity
J3T dog glioma cells (5 × 103/well) were infected with HC-Ad-TK (1 expressing particle/cell) or HC-Ad-β-Gal (1 blue-forming unit/cell). After 48 h, cells were incubated in the presence or absence of the prodrug ganciclovir (GCV, 10 μM) for seven days. HSV1-TK cytotoxicity was also detected in J3T cells infected with HC-Ad-TK (1 transgene-expressing particle/cell) in combination with HC-Ad-mTetON-Flt3L (500 VP/cell). After 48 h, cells were incubated with or without GCV and DOX for seven days. Cell viability was determined by the MTS assay (Promega, Madison, WI, USA) as we previously described.44
Flt3L ELISA
J3T dog glioma cells (5 × 104/well) were infected with HC-Ad-TetON-Flt3L (5,000 VP/cell) alone or in combination with HC-Ad-TK (1 expressing particle/cell) and incubated with or without 1 μg/ml DOX. After 72 h, the supernatants were collected, depleted of cellular debris by centrifugation, and stored at -70°C. Human Flt3L concentration was determined by using a commercial ELISA kit (21-377-296, R&D systems, MN, USA).32
Immunocytochemistry
Transgene expression was determined by immunocytochemistry in J3T cells fixed 72 h after infection (4% paraformaldehyde in phosphate-buffered saline, 15 min, at room temperature) and in dog striatal coronal sections (75 μm) cut with a microtome. Transgenes were detected with anti-β-Gal,35,46 anti-TK,14,15 and anti-Flt3L14,15,47 specific primary antibodies (1:1,000) developed in our laboratory. Inflammatory cells were detected in dog striatal sections treated with trypsin and citrate buffer for antigen retrieval by using monoclonal primary antibodies against dog CD3ϵ (T cells) and CD18 (macrophages and microglia)48 as previously described.32 Antibody binding was revealed by using antirabbit biotinylated secondary antibodies (1:1,000) (Dako, Fort Collins, CO, USA), followed by incubation with peroxidase-conjugated avidin (Vector, Burlingame, CA, USA) and diaminobenzidine (Sigma, St. Louis, MO, USA).
To detect in which cell types the transgenes are expressed, we combined β-Gal, TK, and Flt3L primary antibodies with astrocytic and neuronal cell markers. Guinea pig anti-glial fibrillary astrocytic protein (GFAP, 1:500) (Advanced Immunochemical, Long Beach, CA, USA) was used to detect astrocytes and mouse anti-neuronal nucleus (NeuN) antibody (1:1,000) was used to label mature neuronal cells (Chemicon, Temecula, CA, USA). The fluorescent secondary antibodies (1:1,000) used for detection were goat antirabbit Alexa 488, goat anti-guinea pig Alexa 647, and goat antimouse Alexa 594 (Molecular Probes, Eugene, OR, USA). Nuclei were stained with 4',6-diamidino-2-phenylindole (DAPI) (Molecular Probes/Invitrogen, Carlsbad, CA, USA) and mounted with ProLong Antifade (Molecular Probes/Invitrogen).
Confocal Microscopy
Confocal micrographs were obtained using a Leica confocal microscope TCS SP2 with AOBS equipped with a 405-nm violet-diode UV laser, 488-nm argon laser, and 594- and 633-nm helium-neon lasers; and using a HCX PL APO 63 × 1.4 numerical aperture oil objective (Leica Microsystems Heidelberg, Mannheim, Germany).
Automated Cell Counts
The number of J3T dog glioma cells transduced and the brain area transduced by the adenoviruses in vivo were estimated by automated stereology, using a Zeiss Axio-plan 2 microscope with Ludl electronic MAC 5000 XY stage control (Ludl Electronics Products, Hawthorne, NY, USA) and Axioplan Z-axis control (Carl Zeiss, Thornwood, NY, USA) using the Stereo Investigator software (Microbrightfield, Colchester, VT, USA) as we previously described.32 The area to be sampled was traced with ×1.25 objective. For J3T cell counts, the thickness and the size of the counting frames were 1 μm and 200 × 200 μm, respectively. Selection was made to count cells in 25 sampling sites in each well.
Statistical Analysis
Transduced brain areas and in vitro measurements were analyzed by Student's t-test or two-way analysis of variance (ANOVA), followed by Duncan's test, and automated cell counts were analyzed by chi-square test (NCSS 2004 statistical software). The cutoff point for significance was considered to be p < 0.05. The replicates measured in each cytotoxicity assay consisted of 10 wells/group. Flt3L released was determined in the supernatant of 3 wells/group because of the low variability within replicates in this assay. The β-Gal activity assays were performed using 3 replicates/group. In each in vivo experiment, one dog was injected in five different injection sites by using an identical dose of each Ad used. Experiments were performed at least twice.
Results
mCMV Promoter Elicits Higher Levels of Transgene Expression than the hCMV promoter in Dog Brain In Vivo
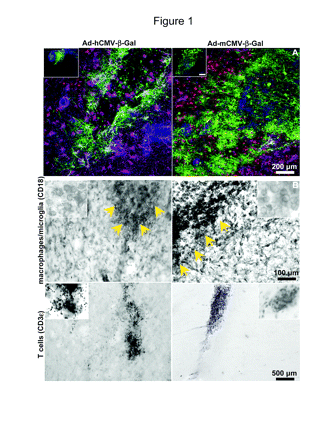
The choice of strong promoters is crucial to exert high transgene expression levels by using lower viral doses, leading to therapeutic efficacy with negligible toxicity. We had previously shown that the mCMV promoter elicited 3-log higher levels of transgene expression within the rodent brain in vivo.30 Thus, we assessed the strength of the mCMV promoter compared with the hCMV promoter in driving transgene expression from adenoviruses in dog brain in vivo. We administered Ads encoding β-Gal under the control of the hCMV promoter or the mCMV promoter by using a dose of 8 × 107 PFU/5 μl per injection site into the cerebral cortex of healthy Beagles. As shown in Fig. 1A, Ad-hCMV-β-Gal elicited an area of transduction of 1.6 mm2 surrounding the injection site, whereas the Ad-mCMV-β-Gal transduced an area of 8 mm2, which was five times larger (p < 0.05, Student's t-test).
Transgene expression (A) and inflammatory cell infiltration (B) in the brain of dogs intracranially injected with adenovirus vectors (Ads) encoding β-galactosidase (β-Gal) under the control of the human cytomegalovirus (hCMV) promoter (Ad-hCMV-β-Gal) or the murine cytomegalovirus (mCMV) promoter (Ad-mCMV-β-Gal). Dogs were injected in the parietal neocortex with Ad-hCMV-β-Gal or Ad-mCMV-β-Gal (8 × 107 PFU in 5 μl per injection site). After seven days, transgene expression and inflammatory cell markers were detected in 75-μm coronal sections. (A) Low-magnification confocal pictures show β-Gal (green) expression in the brain parenchyma surrounding the injection site. Neurons were labeled with anti-neuronal nucleus antibody (red) and astrocytes with anti-glial fibrillary astrocytic protein antibody (magenta). Nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI) (blue). Inset: Lower-magnification images of the injection sites showing β-Gal expression (green) and nuclei stained with DAPI (blue). Scale bar, 500 μm. (B) Macrophages and microglia were detected using an anti-CD18 antibody, and T cells were localized using an anti-CD3ϵ antibody. Arrows indicate the injection site. Inset: High-magnification pictures.
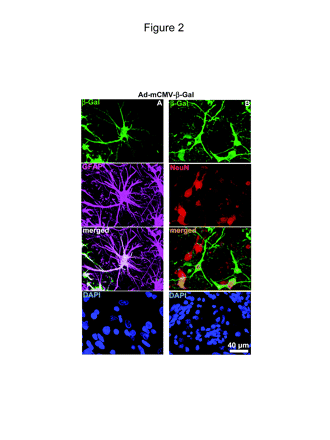
We then determined by immunocytochemistry the cell-type specificity of transgene expression and infiltration of inflammatory cells in the brain of the dogs injected with Ad-mCMV-β-Gal or Ad-hCMV-β-Gal. Although transgene expression was much higher when driven by the mCMV promoter, infiltration of inflammatory cells into the site of injection was similar when dogs were injected with Ad-hCMV-β-Gal and Ad-mCMV-β-Gal (Fig. 1B). Macrophages and microglia were detected through their immunoreactivity for anti-CD18, and T cells were identified by CD3 immunoreactivity. Macrophage infiltration was detected only at the site of Ad injection. We also found T-cell infiltration, although to a lesser extent, which was also localized at the injection site (Fig. 1B, bottom panels). Cell type-specific expression of Ad-encoded transgene in dog brain was detected by double-labeling immunofluorescence using antibodies against GFAP and neuronal cell body (NeuN). Images in Fig. 2 demonstrate that β-Gal encoded within Ad-mCMV-β-Gal is expressed in astrocytes (Fig. 2A) and also in neurons (Fig. 2B).
In vivo transgene expression in astrocytes and neurons of dogs injected with adenovirus vector-murine cytomegalovirus-β-galactosidase (Ad-mCMV-β-Gal). Dogs were injected in the parietal neocortex with Ad-mCMV-β-Gal, 8 × 107 PFU in 5 μl per injection site. After seven days, transgene expression was detected in 75-μm coronal sections. Confocal pictures show neurons, as labeled with anti-neuronal nucleus (NeuN) antibody (red); astrocytes, as detected with anti-glial fibrillary astrocytic protein (GFAP) antibody (magenta); and β-Gal expression (green). Nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI) (blue). (A) Pictures show β-Gal expression from Ad-mCMV-β-Gal in astrocytes (GFAP, magenta). Colocalization of GFAP and β-Gal is depicted in white. (B) Pictures show β-Gal expression from Ad-mCMV-β-Gal in neurons (NeuN, red). Colocalization of NeuN and β-Gal is in orange.
Intracranial Administration of Adenoviruses in Dogs Does Not Induce Adverse Side Effects
We monitored changes in the clinical parameters after the intracranial administration of adenoviruses. Body temperature remained normal during the course of the study in all animals (not shown). Blood counts and serum biochemistry were determined during the surgery, after 24 h, and after seven days. All serum chemistry values (Table 1) and blood count values (Table 2) were within normal range, as were the findings of the urinalysis, which was performed the day of the surgery and after three days (not shown). CSF was collected from the cisterna magna during the surgery and prior to euthanasia. The analysis of the CSF (Table 3) indicated that cellularity was slightly increased seven days after adenovirus administration and was composed of red blood cells along with a population of nucleated cells that included lymphocytes, nondegenerated neutrophils, and small and large mononuclear cells. Other parameters of the CSF, such as color, viscosity, and turbidity, remained normal.
Ranges of serum chemistry values after in vivo administrationa of Ad-hCMV-β-Gal or Ad-mCMV-β-Gal
| . | . | Ad-hCMV-β-Gal . | . | . | Ad-mCMV-β-Gal . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Test . | Ref. Range . | Day 0 . | Day 1 . | Day 7 . | Day 0 . | Day 1 . | Day 7 . | ||||
| Blood urea nitrogen (mg/dl) | 9-31 | 13-14 | 11-14 | 12-16 | 13-17 | 10-19 | 11-15 | ||||
| Creatinine (mg/dl) | 0.6-1.6 | 0.6-0.7 | 0.6-0.7 | 0.7 | 0.6 | 0.6-0.8 | 0.6-0.7 | ||||
| Calcium (mg/dl) | 9.3-11.5 | 10.3-10.4 | 10.2-10.4 | 10.3-10.7 | 9.8-10.8 | 10.2-10.4 | 10.8-11.0 | ||||
| Phosphorus (mg/dl) | 3.3-6.8 | 5.3 | 5.9-6.1 | 5.1-6.0 | 4.9-5.2 | 5.2-6.3 | 4.9-5.3 | ||||
| Magnesium (mg/dl) | 1.7-2.4 | 1.7-1.9 | 1.7 | 1.7-1.8 | 1.8-2.0 | 1.7-1.8 | 1.8-2.0 | ||||
| Protein | 5.0-6.9 | 5.8-6.5 | 5.4-6.3 | 5.8-6.1 | 6.0-6.7 | 6.2-6.3 | 6.2-6.4 | ||||
| Albumin | 2.7-3.7 | 3.1-3.4 | 2.8-3.3 | 3.3-3.4 | 3.2-3.6 | 3.2-3.3 | 3.4-3.6 | ||||
| Globulin | 1.7-3.5 | 2.7-3.1 | 2.6-3.0 | 2.5-2.7 | 2.8-3.1 | 2.9-3.1 | 2.6-3.0 | ||||
| Sodium (mmol/L) | 145-153 | 145-147 | 141-147 | 146-147 | 144-146 | 145-147 | 146-148 | ||||
| Chloride (mmol/L) | 109-118 | 108-110 | 109-110 | 112-113 | 107-108 | 110-111 | 108-109 | ||||
| Potassium (mmol/L) | 3.6-5.3 | 4.0-4.4 | 4.3-4.6 | 4.0-4.6 | 4.0-4.9 | 4.4-4.5 | 4.0-4.9 | ||||
| Bicarbonate (mmol/L) | 15-25 | 20.0-22.0 | 23.0-25.0 | 20.0-23.0 | 19.0-22.0 | 24.0-25.0 | 25.0-26.0 | ||||
| Total bilirubin (mg/dl) | 0-0.3 | 0.2-0.3 | 0.2 | 0.2-0.3 | 0.2-0.3 | 0.2-0.3 | 0.2-0.3 | ||||
| Alkaline phosphatase (U/L) | 8-139 | 64-86 | 84-104 | 72-93 | 57-65 | 63-66 | 49-67 | ||||
| γ-Glutamyltransferase (U/L) | 0-6 | 3 | 3 | 2-3 | <3 | <3 | <3 | ||||
| Alanine aminotransferase (U/L) | 22-92 | 48-54 | 48-61 | 28-49 | 36-41 | 34-41 | 26-30 | ||||
| Glucose (mg/dl) | 75-117 | 42-75 | 89-108 | 100-106 | 48-84 | 82-84 | 83-94 | ||||
| Cholesterol (mg/dl) | 143-373 | 149-171 | 141-160 | 163-185 | 185-208 | 191-201 | 208-219 | ||||
| Amylase (U/L) | 275-1056 | 394-603 | 335-496 | 461-486 | 465-649 | 612-712 | 450-791 | ||||
| . | . | Ad-hCMV-β-Gal . | . | . | Ad-mCMV-β-Gal . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Test . | Ref. Range . | Day 0 . | Day 1 . | Day 7 . | Day 0 . | Day 1 . | Day 7 . | ||||
| Blood urea nitrogen (mg/dl) | 9-31 | 13-14 | 11-14 | 12-16 | 13-17 | 10-19 | 11-15 | ||||
| Creatinine (mg/dl) | 0.6-1.6 | 0.6-0.7 | 0.6-0.7 | 0.7 | 0.6 | 0.6-0.8 | 0.6-0.7 | ||||
| Calcium (mg/dl) | 9.3-11.5 | 10.3-10.4 | 10.2-10.4 | 10.3-10.7 | 9.8-10.8 | 10.2-10.4 | 10.8-11.0 | ||||
| Phosphorus (mg/dl) | 3.3-6.8 | 5.3 | 5.9-6.1 | 5.1-6.0 | 4.9-5.2 | 5.2-6.3 | 4.9-5.3 | ||||
| Magnesium (mg/dl) | 1.7-2.4 | 1.7-1.9 | 1.7 | 1.7-1.8 | 1.8-2.0 | 1.7-1.8 | 1.8-2.0 | ||||
| Protein | 5.0-6.9 | 5.8-6.5 | 5.4-6.3 | 5.8-6.1 | 6.0-6.7 | 6.2-6.3 | 6.2-6.4 | ||||
| Albumin | 2.7-3.7 | 3.1-3.4 | 2.8-3.3 | 3.3-3.4 | 3.2-3.6 | 3.2-3.3 | 3.4-3.6 | ||||
| Globulin | 1.7-3.5 | 2.7-3.1 | 2.6-3.0 | 2.5-2.7 | 2.8-3.1 | 2.9-3.1 | 2.6-3.0 | ||||
| Sodium (mmol/L) | 145-153 | 145-147 | 141-147 | 146-147 | 144-146 | 145-147 | 146-148 | ||||
| Chloride (mmol/L) | 109-118 | 108-110 | 109-110 | 112-113 | 107-108 | 110-111 | 108-109 | ||||
| Potassium (mmol/L) | 3.6-5.3 | 4.0-4.4 | 4.3-4.6 | 4.0-4.6 | 4.0-4.9 | 4.4-4.5 | 4.0-4.9 | ||||
| Bicarbonate (mmol/L) | 15-25 | 20.0-22.0 | 23.0-25.0 | 20.0-23.0 | 19.0-22.0 | 24.0-25.0 | 25.0-26.0 | ||||
| Total bilirubin (mg/dl) | 0-0.3 | 0.2-0.3 | 0.2 | 0.2-0.3 | 0.2-0.3 | 0.2-0.3 | 0.2-0.3 | ||||
| Alkaline phosphatase (U/L) | 8-139 | 64-86 | 84-104 | 72-93 | 57-65 | 63-66 | 49-67 | ||||
| γ-Glutamyltransferase (U/L) | 0-6 | 3 | 3 | 2-3 | <3 | <3 | <3 | ||||
| Alanine aminotransferase (U/L) | 22-92 | 48-54 | 48-61 | 28-49 | 36-41 | 34-41 | 26-30 | ||||
| Glucose (mg/dl) | 75-117 | 42-75 | 89-108 | 100-106 | 48-84 | 82-84 | 83-94 | ||||
| Cholesterol (mg/dl) | 143-373 | 149-171 | 141-160 | 163-185 | 185-208 | 191-201 | 208-219 | ||||
| Amylase (U/L) | 275-1056 | 394-603 | 335-496 | 461-486 | 465-649 | 612-712 | 450-791 | ||||
Abbreviations: Ad-hCMV-β-Gal, adenovirus vector-human cytomegalovirus promoter-β-galactosidase; Ad-mCMV-β-Gal, adenovirus vector-murine cytomegalovirus promoter-β-galactosidase.
Total n = 2 dogs/group.
Ranges of serum chemistry values after in vivo administrationa of Ad-hCMV-β-Gal or Ad-mCMV-β-Gal
| . | . | Ad-hCMV-β-Gal . | . | . | Ad-mCMV-β-Gal . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Test . | Ref. Range . | Day 0 . | Day 1 . | Day 7 . | Day 0 . | Day 1 . | Day 7 . | ||||
| Blood urea nitrogen (mg/dl) | 9-31 | 13-14 | 11-14 | 12-16 | 13-17 | 10-19 | 11-15 | ||||
| Creatinine (mg/dl) | 0.6-1.6 | 0.6-0.7 | 0.6-0.7 | 0.7 | 0.6 | 0.6-0.8 | 0.6-0.7 | ||||
| Calcium (mg/dl) | 9.3-11.5 | 10.3-10.4 | 10.2-10.4 | 10.3-10.7 | 9.8-10.8 | 10.2-10.4 | 10.8-11.0 | ||||
| Phosphorus (mg/dl) | 3.3-6.8 | 5.3 | 5.9-6.1 | 5.1-6.0 | 4.9-5.2 | 5.2-6.3 | 4.9-5.3 | ||||
| Magnesium (mg/dl) | 1.7-2.4 | 1.7-1.9 | 1.7 | 1.7-1.8 | 1.8-2.0 | 1.7-1.8 | 1.8-2.0 | ||||
| Protein | 5.0-6.9 | 5.8-6.5 | 5.4-6.3 | 5.8-6.1 | 6.0-6.7 | 6.2-6.3 | 6.2-6.4 | ||||
| Albumin | 2.7-3.7 | 3.1-3.4 | 2.8-3.3 | 3.3-3.4 | 3.2-3.6 | 3.2-3.3 | 3.4-3.6 | ||||
| Globulin | 1.7-3.5 | 2.7-3.1 | 2.6-3.0 | 2.5-2.7 | 2.8-3.1 | 2.9-3.1 | 2.6-3.0 | ||||
| Sodium (mmol/L) | 145-153 | 145-147 | 141-147 | 146-147 | 144-146 | 145-147 | 146-148 | ||||
| Chloride (mmol/L) | 109-118 | 108-110 | 109-110 | 112-113 | 107-108 | 110-111 | 108-109 | ||||
| Potassium (mmol/L) | 3.6-5.3 | 4.0-4.4 | 4.3-4.6 | 4.0-4.6 | 4.0-4.9 | 4.4-4.5 | 4.0-4.9 | ||||
| Bicarbonate (mmol/L) | 15-25 | 20.0-22.0 | 23.0-25.0 | 20.0-23.0 | 19.0-22.0 | 24.0-25.0 | 25.0-26.0 | ||||
| Total bilirubin (mg/dl) | 0-0.3 | 0.2-0.3 | 0.2 | 0.2-0.3 | 0.2-0.3 | 0.2-0.3 | 0.2-0.3 | ||||
| Alkaline phosphatase (U/L) | 8-139 | 64-86 | 84-104 | 72-93 | 57-65 | 63-66 | 49-67 | ||||
| γ-Glutamyltransferase (U/L) | 0-6 | 3 | 3 | 2-3 | <3 | <3 | <3 | ||||
| Alanine aminotransferase (U/L) | 22-92 | 48-54 | 48-61 | 28-49 | 36-41 | 34-41 | 26-30 | ||||
| Glucose (mg/dl) | 75-117 | 42-75 | 89-108 | 100-106 | 48-84 | 82-84 | 83-94 | ||||
| Cholesterol (mg/dl) | 143-373 | 149-171 | 141-160 | 163-185 | 185-208 | 191-201 | 208-219 | ||||
| Amylase (U/L) | 275-1056 | 394-603 | 335-496 | 461-486 | 465-649 | 612-712 | 450-791 | ||||
| . | . | Ad-hCMV-β-Gal . | . | . | Ad-mCMV-β-Gal . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Test . | Ref. Range . | Day 0 . | Day 1 . | Day 7 . | Day 0 . | Day 1 . | Day 7 . | ||||
| Blood urea nitrogen (mg/dl) | 9-31 | 13-14 | 11-14 | 12-16 | 13-17 | 10-19 | 11-15 | ||||
| Creatinine (mg/dl) | 0.6-1.6 | 0.6-0.7 | 0.6-0.7 | 0.7 | 0.6 | 0.6-0.8 | 0.6-0.7 | ||||
| Calcium (mg/dl) | 9.3-11.5 | 10.3-10.4 | 10.2-10.4 | 10.3-10.7 | 9.8-10.8 | 10.2-10.4 | 10.8-11.0 | ||||
| Phosphorus (mg/dl) | 3.3-6.8 | 5.3 | 5.9-6.1 | 5.1-6.0 | 4.9-5.2 | 5.2-6.3 | 4.9-5.3 | ||||
| Magnesium (mg/dl) | 1.7-2.4 | 1.7-1.9 | 1.7 | 1.7-1.8 | 1.8-2.0 | 1.7-1.8 | 1.8-2.0 | ||||
| Protein | 5.0-6.9 | 5.8-6.5 | 5.4-6.3 | 5.8-6.1 | 6.0-6.7 | 6.2-6.3 | 6.2-6.4 | ||||
| Albumin | 2.7-3.7 | 3.1-3.4 | 2.8-3.3 | 3.3-3.4 | 3.2-3.6 | 3.2-3.3 | 3.4-3.6 | ||||
| Globulin | 1.7-3.5 | 2.7-3.1 | 2.6-3.0 | 2.5-2.7 | 2.8-3.1 | 2.9-3.1 | 2.6-3.0 | ||||
| Sodium (mmol/L) | 145-153 | 145-147 | 141-147 | 146-147 | 144-146 | 145-147 | 146-148 | ||||
| Chloride (mmol/L) | 109-118 | 108-110 | 109-110 | 112-113 | 107-108 | 110-111 | 108-109 | ||||
| Potassium (mmol/L) | 3.6-5.3 | 4.0-4.4 | 4.3-4.6 | 4.0-4.6 | 4.0-4.9 | 4.4-4.5 | 4.0-4.9 | ||||
| Bicarbonate (mmol/L) | 15-25 | 20.0-22.0 | 23.0-25.0 | 20.0-23.0 | 19.0-22.0 | 24.0-25.0 | 25.0-26.0 | ||||
| Total bilirubin (mg/dl) | 0-0.3 | 0.2-0.3 | 0.2 | 0.2-0.3 | 0.2-0.3 | 0.2-0.3 | 0.2-0.3 | ||||
| Alkaline phosphatase (U/L) | 8-139 | 64-86 | 84-104 | 72-93 | 57-65 | 63-66 | 49-67 | ||||
| γ-Glutamyltransferase (U/L) | 0-6 | 3 | 3 | 2-3 | <3 | <3 | <3 | ||||
| Alanine aminotransferase (U/L) | 22-92 | 48-54 | 48-61 | 28-49 | 36-41 | 34-41 | 26-30 | ||||
| Glucose (mg/dl) | 75-117 | 42-75 | 89-108 | 100-106 | 48-84 | 82-84 | 83-94 | ||||
| Cholesterol (mg/dl) | 143-373 | 149-171 | 141-160 | 163-185 | 185-208 | 191-201 | 208-219 | ||||
| Amylase (U/L) | 275-1056 | 394-603 | 335-496 | 461-486 | 465-649 | 612-712 | 450-791 | ||||
Abbreviations: Ad-hCMV-β-Gal, adenovirus vector-human cytomegalovirus promoter-β-galactosidase; Ad-mCMV-β-Gal, adenovirus vector-murine cytomegalovirus promoter-β-galactosidase.
Total n = 2 dogs/group.
Ranges of complete blood counts after in vivo administration of Ad-hCMV-β-Gal or Ad-mCMV-β-Gal
| . | . | Ad-hCMV-β-Gal . | . | . | Ad-mCMV-β-Gal . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Test . | Ref. Range . | Day 0 . | Day 1 . | Day 7 . | Day 0 . | Day 1 . | Day 7 . | ||||
| White blood counts (×103/μl) | 4.1-13.3 | 8.4-8.8 | 11.7 | 7.8-8.3 | 7.5-13.2 | 7.7-11.0 | 10.7 | ||||
| Segmented neutrophils (×103/μl) | 2.1-11.2 | 5.88-7.04 | 6.90-7.72 | 5.64-6.01 | 5.48-10.03 | 4.85-6.27 | 8.88-9.20 | ||||
| Band neutrophils (×103/μl) | 0-0.13 | 0.00-0.08 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | ||||
| Lymphocytes (×103/μl) | 0.78-3.36 | 1.23-1.34 | 3.04-3.63 | 1.33-1.99 | 1.50-2.24 | 2.00-2.97 | 0.86-1.28 | ||||
| Mononuclear cells (×103/μl) | 0-1.2 | 0.53-0.84 | 0.59-0.70 | 0.47-0.50 | 0.53-0.92 | 0.62-1.32 | 0.32-0.64 | ||||
| Eosinophils (×103/μl) | 0-1.2 | 0.00-0.25 | 0.35-0.47 | 0.00-0.17 | 0.0 | 0.23-0.44 | 0.00-0.21 | ||||
| Basophils (×103/μl) | 0-0.13 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | ||||
| Red blood cells (×106/μl) | 5.71-88.29 | 5.79-6.59 | 5.96-6.06 | 6.68-6.83 | 6.48-6.72 | 6.32-6.89 | 6.60-7.11 | ||||
| Hemoglobin (g/dl) | 13.5-19.9 | 14.1-15.1 | 13.9-14.8 | 15.5-16.2 | 15.6-16.4 | 15.1-16.6 | 15.9-16.9 | ||||
| Hematocrit (%) | 38.5-56.7 | 40.6-44.0 | 40.0-41.2 | 45.1-47.3 | 44.7-46.7 | 43.5-47.6 | 45.7-48.8 | ||||
| Mean cell volume (fl) | 64-73 | 66.7-70.1 | 66.0-69.2 | 66.0-70.9 | 68.9-69.5 | 68.8-69.0 | 68.6-69.2 | ||||
| Mean cell hemoglobin (pg) | 21.8-26 | 22.9-24.4 | 23.0-24.9 | 22.7-24.3 | 24.1-24.4 | 24.0-24.1 | 23.7-24.1 | ||||
| Mean cell hemoglobin concentration (g/dl) | 33.6-36.6 | 34.4-34.8 | 34.8-36.0 | 34.3-34.4 | 35.0-35.1 | 34.9 | 34.6-34.8 | ||||
| Red cell distributive width (%) | 12.5-16.5 | 14.0-14.3 | 14.7-14.9 | 15.2-16.2 | 14.5-16.4 | 14.6-14.8 | 15.7-16.0 | ||||
| Platelet counts (×103/μl) | 160-425 | 170-199 | 183-353 | 224-304 | 300-423 | 321-425 | 181-429 | ||||
| Mean platelet volume (fl) | 6-11 | 10.9 | 7.4-12.9 | 7.7-8.7 | 8.3 | 7.5-8.8 | 8.6-10.3 | ||||
| Plateletcrit (%) | 0.11-0.35 | 0.11 | 0.11-0.26 | 0.20-0.23 | 0.25-0.39 | 0.24-0.40 | 0.19-0.37 | ||||
| Platelet distribution width (%) | 15.3-18.1 | 17.9 | 16.8-18.3 | 16.8-17.2 | 16.5-17.5 | 17.0-17.3 | 17.2-20.5 | ||||
| Thiamine pyrophosphate (g/dl) | 5.8-7.2 | 6.1-6.3 | 6.0-6.8 | 6.2-6.9 | 6.6-7.1 | 6.7 | 6.3-6.6 | ||||
| . | . | Ad-hCMV-β-Gal . | . | . | Ad-mCMV-β-Gal . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Test . | Ref. Range . | Day 0 . | Day 1 . | Day 7 . | Day 0 . | Day 1 . | Day 7 . | ||||
| White blood counts (×103/μl) | 4.1-13.3 | 8.4-8.8 | 11.7 | 7.8-8.3 | 7.5-13.2 | 7.7-11.0 | 10.7 | ||||
| Segmented neutrophils (×103/μl) | 2.1-11.2 | 5.88-7.04 | 6.90-7.72 | 5.64-6.01 | 5.48-10.03 | 4.85-6.27 | 8.88-9.20 | ||||
| Band neutrophils (×103/μl) | 0-0.13 | 0.00-0.08 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | ||||
| Lymphocytes (×103/μl) | 0.78-3.36 | 1.23-1.34 | 3.04-3.63 | 1.33-1.99 | 1.50-2.24 | 2.00-2.97 | 0.86-1.28 | ||||
| Mononuclear cells (×103/μl) | 0-1.2 | 0.53-0.84 | 0.59-0.70 | 0.47-0.50 | 0.53-0.92 | 0.62-1.32 | 0.32-0.64 | ||||
| Eosinophils (×103/μl) | 0-1.2 | 0.00-0.25 | 0.35-0.47 | 0.00-0.17 | 0.0 | 0.23-0.44 | 0.00-0.21 | ||||
| Basophils (×103/μl) | 0-0.13 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | ||||
| Red blood cells (×106/μl) | 5.71-88.29 | 5.79-6.59 | 5.96-6.06 | 6.68-6.83 | 6.48-6.72 | 6.32-6.89 | 6.60-7.11 | ||||
| Hemoglobin (g/dl) | 13.5-19.9 | 14.1-15.1 | 13.9-14.8 | 15.5-16.2 | 15.6-16.4 | 15.1-16.6 | 15.9-16.9 | ||||
| Hematocrit (%) | 38.5-56.7 | 40.6-44.0 | 40.0-41.2 | 45.1-47.3 | 44.7-46.7 | 43.5-47.6 | 45.7-48.8 | ||||
| Mean cell volume (fl) | 64-73 | 66.7-70.1 | 66.0-69.2 | 66.0-70.9 | 68.9-69.5 | 68.8-69.0 | 68.6-69.2 | ||||
| Mean cell hemoglobin (pg) | 21.8-26 | 22.9-24.4 | 23.0-24.9 | 22.7-24.3 | 24.1-24.4 | 24.0-24.1 | 23.7-24.1 | ||||
| Mean cell hemoglobin concentration (g/dl) | 33.6-36.6 | 34.4-34.8 | 34.8-36.0 | 34.3-34.4 | 35.0-35.1 | 34.9 | 34.6-34.8 | ||||
| Red cell distributive width (%) | 12.5-16.5 | 14.0-14.3 | 14.7-14.9 | 15.2-16.2 | 14.5-16.4 | 14.6-14.8 | 15.7-16.0 | ||||
| Platelet counts (×103/μl) | 160-425 | 170-199 | 183-353 | 224-304 | 300-423 | 321-425 | 181-429 | ||||
| Mean platelet volume (fl) | 6-11 | 10.9 | 7.4-12.9 | 7.7-8.7 | 8.3 | 7.5-8.8 | 8.6-10.3 | ||||
| Plateletcrit (%) | 0.11-0.35 | 0.11 | 0.11-0.26 | 0.20-0.23 | 0.25-0.39 | 0.24-0.40 | 0.19-0.37 | ||||
| Platelet distribution width (%) | 15.3-18.1 | 17.9 | 16.8-18.3 | 16.8-17.2 | 16.5-17.5 | 17.0-17.3 | 17.2-20.5 | ||||
| Thiamine pyrophosphate (g/dl) | 5.8-7.2 | 6.1-6.3 | 6.0-6.8 | 6.2-6.9 | 6.6-7.1 | 6.7 | 6.3-6.6 | ||||
Abbreviations: Ad-hCMV-β-Gal, adenovirus vector-human cytomegalovirus promoter-β-galactosidase; Ad-mCMV-β-Gal, adenovirus vector-murine cytomegalovirus promoter-β-galactosidase.
Ranges of complete blood counts after in vivo administration of Ad-hCMV-β-Gal or Ad-mCMV-β-Gal
| . | . | Ad-hCMV-β-Gal . | . | . | Ad-mCMV-β-Gal . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Test . | Ref. Range . | Day 0 . | Day 1 . | Day 7 . | Day 0 . | Day 1 . | Day 7 . | ||||
| White blood counts (×103/μl) | 4.1-13.3 | 8.4-8.8 | 11.7 | 7.8-8.3 | 7.5-13.2 | 7.7-11.0 | 10.7 | ||||
| Segmented neutrophils (×103/μl) | 2.1-11.2 | 5.88-7.04 | 6.90-7.72 | 5.64-6.01 | 5.48-10.03 | 4.85-6.27 | 8.88-9.20 | ||||
| Band neutrophils (×103/μl) | 0-0.13 | 0.00-0.08 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | ||||
| Lymphocytes (×103/μl) | 0.78-3.36 | 1.23-1.34 | 3.04-3.63 | 1.33-1.99 | 1.50-2.24 | 2.00-2.97 | 0.86-1.28 | ||||
| Mononuclear cells (×103/μl) | 0-1.2 | 0.53-0.84 | 0.59-0.70 | 0.47-0.50 | 0.53-0.92 | 0.62-1.32 | 0.32-0.64 | ||||
| Eosinophils (×103/μl) | 0-1.2 | 0.00-0.25 | 0.35-0.47 | 0.00-0.17 | 0.0 | 0.23-0.44 | 0.00-0.21 | ||||
| Basophils (×103/μl) | 0-0.13 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | ||||
| Red blood cells (×106/μl) | 5.71-88.29 | 5.79-6.59 | 5.96-6.06 | 6.68-6.83 | 6.48-6.72 | 6.32-6.89 | 6.60-7.11 | ||||
| Hemoglobin (g/dl) | 13.5-19.9 | 14.1-15.1 | 13.9-14.8 | 15.5-16.2 | 15.6-16.4 | 15.1-16.6 | 15.9-16.9 | ||||
| Hematocrit (%) | 38.5-56.7 | 40.6-44.0 | 40.0-41.2 | 45.1-47.3 | 44.7-46.7 | 43.5-47.6 | 45.7-48.8 | ||||
| Mean cell volume (fl) | 64-73 | 66.7-70.1 | 66.0-69.2 | 66.0-70.9 | 68.9-69.5 | 68.8-69.0 | 68.6-69.2 | ||||
| Mean cell hemoglobin (pg) | 21.8-26 | 22.9-24.4 | 23.0-24.9 | 22.7-24.3 | 24.1-24.4 | 24.0-24.1 | 23.7-24.1 | ||||
| Mean cell hemoglobin concentration (g/dl) | 33.6-36.6 | 34.4-34.8 | 34.8-36.0 | 34.3-34.4 | 35.0-35.1 | 34.9 | 34.6-34.8 | ||||
| Red cell distributive width (%) | 12.5-16.5 | 14.0-14.3 | 14.7-14.9 | 15.2-16.2 | 14.5-16.4 | 14.6-14.8 | 15.7-16.0 | ||||
| Platelet counts (×103/μl) | 160-425 | 170-199 | 183-353 | 224-304 | 300-423 | 321-425 | 181-429 | ||||
| Mean platelet volume (fl) | 6-11 | 10.9 | 7.4-12.9 | 7.7-8.7 | 8.3 | 7.5-8.8 | 8.6-10.3 | ||||
| Plateletcrit (%) | 0.11-0.35 | 0.11 | 0.11-0.26 | 0.20-0.23 | 0.25-0.39 | 0.24-0.40 | 0.19-0.37 | ||||
| Platelet distribution width (%) | 15.3-18.1 | 17.9 | 16.8-18.3 | 16.8-17.2 | 16.5-17.5 | 17.0-17.3 | 17.2-20.5 | ||||
| Thiamine pyrophosphate (g/dl) | 5.8-7.2 | 6.1-6.3 | 6.0-6.8 | 6.2-6.9 | 6.6-7.1 | 6.7 | 6.3-6.6 | ||||
| . | . | Ad-hCMV-β-Gal . | . | . | Ad-mCMV-β-Gal . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Test . | Ref. Range . | Day 0 . | Day 1 . | Day 7 . | Day 0 . | Day 1 . | Day 7 . | ||||
| White blood counts (×103/μl) | 4.1-13.3 | 8.4-8.8 | 11.7 | 7.8-8.3 | 7.5-13.2 | 7.7-11.0 | 10.7 | ||||
| Segmented neutrophils (×103/μl) | 2.1-11.2 | 5.88-7.04 | 6.90-7.72 | 5.64-6.01 | 5.48-10.03 | 4.85-6.27 | 8.88-9.20 | ||||
| Band neutrophils (×103/μl) | 0-0.13 | 0.00-0.08 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | ||||
| Lymphocytes (×103/μl) | 0.78-3.36 | 1.23-1.34 | 3.04-3.63 | 1.33-1.99 | 1.50-2.24 | 2.00-2.97 | 0.86-1.28 | ||||
| Mononuclear cells (×103/μl) | 0-1.2 | 0.53-0.84 | 0.59-0.70 | 0.47-0.50 | 0.53-0.92 | 0.62-1.32 | 0.32-0.64 | ||||
| Eosinophils (×103/μl) | 0-1.2 | 0.00-0.25 | 0.35-0.47 | 0.00-0.17 | 0.0 | 0.23-0.44 | 0.00-0.21 | ||||
| Basophils (×103/μl) | 0-0.13 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | ||||
| Red blood cells (×106/μl) | 5.71-88.29 | 5.79-6.59 | 5.96-6.06 | 6.68-6.83 | 6.48-6.72 | 6.32-6.89 | 6.60-7.11 | ||||
| Hemoglobin (g/dl) | 13.5-19.9 | 14.1-15.1 | 13.9-14.8 | 15.5-16.2 | 15.6-16.4 | 15.1-16.6 | 15.9-16.9 | ||||
| Hematocrit (%) | 38.5-56.7 | 40.6-44.0 | 40.0-41.2 | 45.1-47.3 | 44.7-46.7 | 43.5-47.6 | 45.7-48.8 | ||||
| Mean cell volume (fl) | 64-73 | 66.7-70.1 | 66.0-69.2 | 66.0-70.9 | 68.9-69.5 | 68.8-69.0 | 68.6-69.2 | ||||
| Mean cell hemoglobin (pg) | 21.8-26 | 22.9-24.4 | 23.0-24.9 | 22.7-24.3 | 24.1-24.4 | 24.0-24.1 | 23.7-24.1 | ||||
| Mean cell hemoglobin concentration (g/dl) | 33.6-36.6 | 34.4-34.8 | 34.8-36.0 | 34.3-34.4 | 35.0-35.1 | 34.9 | 34.6-34.8 | ||||
| Red cell distributive width (%) | 12.5-16.5 | 14.0-14.3 | 14.7-14.9 | 15.2-16.2 | 14.5-16.4 | 14.6-14.8 | 15.7-16.0 | ||||
| Platelet counts (×103/μl) | 160-425 | 170-199 | 183-353 | 224-304 | 300-423 | 321-425 | 181-429 | ||||
| Mean platelet volume (fl) | 6-11 | 10.9 | 7.4-12.9 | 7.7-8.7 | 8.3 | 7.5-8.8 | 8.6-10.3 | ||||
| Plateletcrit (%) | 0.11-0.35 | 0.11 | 0.11-0.26 | 0.20-0.23 | 0.25-0.39 | 0.24-0.40 | 0.19-0.37 | ||||
| Platelet distribution width (%) | 15.3-18.1 | 17.9 | 16.8-18.3 | 16.8-17.2 | 16.5-17.5 | 17.0-17.3 | 17.2-20.5 | ||||
| Thiamine pyrophosphate (g/dl) | 5.8-7.2 | 6.1-6.3 | 6.0-6.8 | 6.2-6.9 | 6.6-7.1 | 6.7 | 6.3-6.6 | ||||
Abbreviations: Ad-hCMV-β-Gal, adenovirus vector-human cytomegalovirus promoter-β-galactosidase; Ad-mCMV-β-Gal, adenovirus vector-murine cytomegalovirus promoter-β-galactosidase.
Cerebrospinal fluid analysis seven days after in vivo administration of Ad-hCMV-β-Gal or Ad-mCMV-β-Gal
| . | . | Ad-hCMV-β-Gal . | Ad-mCMV-β-Gal . |
|---|---|---|---|
| Parameter . | Ref. Range . | Day 7 . | Day 7 . |
| Color | Colorless | Colorless | Colorless |
| Turbidity | Clear | Clear | Clear-turbid |
| Viscosity | Low | Low | Low |
| Volume (ml) | 1.5-2.0 | 1.0-1.8 | |
| Nucleated cells/μl | <5 | 24-40 | 3-361 |
| Red blood cells/μl | <200 | 8-162 | 118-1,238 |
| Diagnosis | Mononuclear pleocytosis | Mixed-cell pleocytosis |
| . | . | Ad-hCMV-β-Gal . | Ad-mCMV-β-Gal . |
|---|---|---|---|
| Parameter . | Ref. Range . | Day 7 . | Day 7 . |
| Color | Colorless | Colorless | Colorless |
| Turbidity | Clear | Clear | Clear-turbid |
| Viscosity | Low | Low | Low |
| Volume (ml) | 1.5-2.0 | 1.0-1.8 | |
| Nucleated cells/μl | <5 | 24-40 | 3-361 |
| Red blood cells/μl | <200 | 8-162 | 118-1,238 |
| Diagnosis | Mononuclear pleocytosis | Mixed-cell pleocytosis |
Abbreviations: Ad-hCMV-β-Gal, adenovirus vector-human cytomegalovirus promoter-β-galactosidase; Ad-mCMV-β-Gal, adenovirus vector-murine cytomegalovirus promoter-β-galactosidase.
Cerebrospinal fluid analysis seven days after in vivo administration of Ad-hCMV-β-Gal or Ad-mCMV-β-Gal
| . | . | Ad-hCMV-β-Gal . | Ad-mCMV-β-Gal . |
|---|---|---|---|
| Parameter . | Ref. Range . | Day 7 . | Day 7 . |
| Color | Colorless | Colorless | Colorless |
| Turbidity | Clear | Clear | Clear-turbid |
| Viscosity | Low | Low | Low |
| Volume (ml) | 1.5-2.0 | 1.0-1.8 | |
| Nucleated cells/μl | <5 | 24-40 | 3-361 |
| Red blood cells/μl | <200 | 8-162 | 118-1,238 |
| Diagnosis | Mononuclear pleocytosis | Mixed-cell pleocytosis |
| . | . | Ad-hCMV-β-Gal . | Ad-mCMV-β-Gal . |
|---|---|---|---|
| Parameter . | Ref. Range . | Day 7 . | Day 7 . |
| Color | Colorless | Colorless | Colorless |
| Turbidity | Clear | Clear | Clear-turbid |
| Viscosity | Low | Low | Low |
| Volume (ml) | 1.5-2.0 | 1.0-1.8 | |
| Nucleated cells/μl | <5 | 24-40 | 3-361 |
| Red blood cells/μl | <200 | 8-162 | 118-1,238 |
| Diagnosis | Mononuclear pleocytosis | Mixed-cell pleocytosis |
Abbreviations: Ad-hCMV-β-Gal, adenovirus vector-human cytomegalovirus promoter-β-galactosidase; Ad-mCMV-β-Gal, adenovirus vector-murine cytomegalovirus promoter-β-galactosidase.
mCMV Promoter Elicits Higher Levels of Transgene Expression in the Context of HC-Ads in Dog Glioma Cells In Vitro
Since we aim to develop Ad-mediated gene-therapeutic approaches in dogs bearing spontaneous GBM, we determined whether the mCMV promoter also yields higher levels of transgene expression than the hCMV promoter in dog glioma cells in vitro. J3T dog glioma cells were infected with 30 PFU/cell of Ad-hCMV-β-Gal or Ad-mCMV-β-Gal for 72 h. Transgene expression was detected by β-Gal activity assayed in total cell protein extracts. Although β-Gal expression was readily detected in J3T cells infected with both vectors, β-Gal enzymatic activity was more than 30 times higher (p < 0.05) when cells were infected with Ad-mCMV-β-Gal (2.16 ± 0.01 enzymatic activity/min) than when they were infected with Ad-hCMV-β-Gal (0.065 ± 0.003 enzymatic activity/min). Thus, we used the mCMV to drive the expression of the therapeutic genes, i.e., HSV1-TK and Flt3L within HC-Ads.
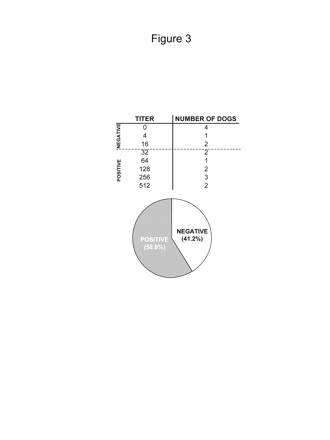
Outbred dogs might exhibit preexisting immunity against adenoviruses, which would hamper long-term transgene expression from first-generation Ads and lead to severe inflammatory side effects.17,34-36,43,49-51 We therefore determined the prevalence of antiadenoviral neutralizing antibodies in the serum from 17 outbred dogs recruited at the College of Veterinary Medicine, University of Minnesota and the Department of Comparative Medicine at Cedars-Sinai Medical Center (Fig. 3). Titers that were lower than 1/16 were considered background, as is conventionally accepted for human anti-Ad titers.37 We determined that 60% of the outbred dogs exhibited neutralizing antibodies against adenovirus, with an average titer of 1/257 ± 56. Considering that preexisting immunity against adenoviruses can impair long-term transgene expression and trigger immune/inflammatory processes,33-36,50,51 HC-Ads31,38,40,50,52,53 will be required for the treatment of GBM in dogs and humans.
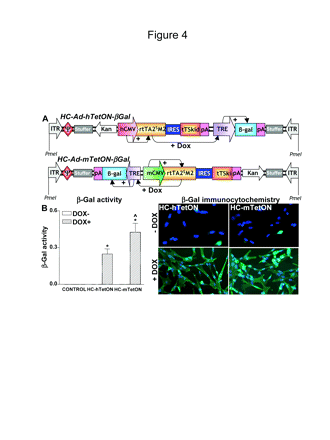
Since levels of transgene expression may depend on the viral vector backbone, we compared the ability of the mCMV promoter and the hCMV promoter in the context of a regulatable HC-Ads expressing β-Gal in dog J3T glioma cells. Cells were infected with regulatable HC-Ads expressing β-Gal under the control of the TetON system driven by the mCMV promoter (HC-Ad-mTetON-β-Gal) or the hCMV promoter (HC-Ad-hTetON-β-Gal) (Fig. 4A). To activate transgene expression, the cells were incubated in the presence of DOX (1 μg/ml) for 72 h. Transgene expression was assessed by immunocytochemistry and β-Gal enzymatic activity. As shown in Fig. 4B, when cells were incubated in the absence of DOX, β-Gal staining was detected only sporadically, whereas in the presence of the inducer, both vectors yielded β-Gal expression in almost the totality of cells. Tallying with our previous data in human glioma cells,32 β-Gal activity in J3T dog glioma cells was 70% higher when the TetON system was driven by the mCMV promoter compared with the hCMV promoter (Fig. 4B).
Levels of neutralizing anti-adenovirus vector (Ad) antibodies in outbred dogs. Sera from healthy outbred dogs recruited at the College of Veterinary Sciences, University of Minnesota, and the Department of Comparative Medicine at Cedars-Sinai Medical Center, Los Angeles (n = 17) were heat inactivated and used to detect antiadenoviral neutralizing antibodies. Samples were considered positive when titers were above 1/16.
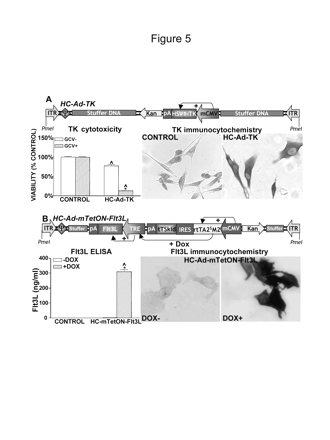
HC-Ads Encoding the Therapeutic Transgenes HSV1-TK and Flt3L Successfully Transduce Dog Glioma Cells In Vitro and Exert Antitumoral Effects
We previously demonstrated that an immunotherapeutic approach that combines the cytotoxic properties of HSV1-TK, which kills glioma cells in the presence of ganciclovir (GCV),32,42,54 with the immunostimulatory effects of Flt3L14,15,47 is very efficient in eliminating large, intracranial GBM in the syngeneic Lewis rat model.15 We therefore tested the ability of HC-Ads encoding the therapeutic transgenes in dog GBM cells. An HC-Ad encoding HSV1-TK under the control of the mCMV promoter was used to infect J3T dog glioma cells at a dose of 1 transgene-expressing particle (TEP) per cell (Fig. 5A). After 48 h, transgene expression was readily detected by immunocytochemistry. The antitumoral effect of TK was induced by incubating the cells in the presence of the prodrug GCV for seven days. Incubation of cells with 10 μM of GCV had no cytotoxic effects per se. However, incubation with GCV after HC-Ad-TK infection exerted potent cytotoxicity, with 90% cell death. Expression of HSV1-TK in the absence of GCV caused low levels of cytotoxicity (approx. 20%). To determine whether the cytotoxic effects of HC-Ad-TK were transgene specific and not induced by HC-Ad infection, J3T cells were infected with a HC-Ad encoding β-Gal under the control of the mCMV promoter (HC-Ad-β-Gal) at the same dose as HC-Ad-TK in the presence or absence of GCV (not shown). HC-Ad-β-Gal did not modify the percentage of cell death, even in the presence of GCV. We conclude that the toxic effects seen were caused by expression of HSV1-TK and not by HC-Ad infection of J3T cells.
In vitro expression of β-galactosidase (β-Gal) in J3T dog glioma cells infected with high-capacity adenovirus vectors (HC-Ads). (A) Linear depiction of the HC-Ad encoding the β-Gal transgene and human cytomegalovirus (hCMV)- or murine cytomegalovirus (mCMV)-driven regulatable tetracycline-dependent (TetON) switch cassette. The constructs indicate the individual components and the orientation of the cassettes and their promoters. The hCMV promoter (top panel) or the mCMV promoter (bottom panel) drives the expression of the tetracycline-dependent transactivator (rtTA2SM2), which in the presence of doxycycline (DOX) activates the tetracycline-responsive element (TRE) promoter to drive the expression of the β-Gal gene. (B) J3T dog glioma cells were infected with 30 BFU/cell of HC-Ads encoding β-Gal under the control of a TetON system driven by the hCMV (HC-Ad-hTetON-β-Gal) or the mCMV (HC-Ad-mTe-tON-β-Gal). Cells were incubated in the presence or absence of DOX (1 μg/ml) and, after 72 h, transgene expression was determined by immunocytochemistry (right panels) and β-Gal activity assay (left panel). Columns represent the mean ± SEM of β-Gal activity (n= 3 wells/group), calculated as o-nitrophenol produced (mg/ml)/sample protein content (mg/ml)/incubation time (min). (*p < 0.001 DOX+ vs. DOX-; ^p < 0.005 vs. HC-Ad-hTetON-β-Gal.) Two-way analysis of variance (ANOVA) followed by Duncan's test.
An HC-Ad encoding Flt3L under the control of the TetON system driven by the mCMV promoter was used to infect J3T dog glioma cells (Fig. 5B). The cells were infected for 48 h in the presence or absence of DOX. Transgene expression was detected by immunocytochemistry and ELISA. The images in Fig. 5B indicate that transgene expression was detected only when cells were incubated in the presence of the inducer. Stringent control of Flt3L release was also assessed by ELISA. In the presence of DOX, 7 nM of Flt3L was detected in the supernatant of these cultures. Expression of Flt3L in the absence of DOX (off state) was around 0.1% compared to its expression in the on state (Fig. 5B).
Since the anti-glioma therapeutic approach entails the delivery of both transgenes, TK and Flt3L, we infected J3T dog glioma cells with HC-Ad-TK and/or HC-Ad-mTetON-Flt3L in the presence or absence of DOX. After 48 h, we detected the expression of both transgenes by immunocytochemistry and ELISA. TK cytotoxicity was determined by the MTS assay after the incubation of cells with GCV (10 μM) and DOX for an additional 48 h. Transduction efficiency of HC-Ad-TK (Fig. 6A), as assessed by immunocytochemistry, was similar when J3T cells were infected with the vector alone or combined with HC-Ad-mTetON-Flt3L (approx. 25%). In the absence of GCV, coinfection with both vectors did not modify the viability of J3T cells. The cytotoxic effect of HC-Ad-TK/GCV observed in the presence of GCV was unaffected by coinfection with HC-Ad-mTetON-Flt3L (Fig. 6A).
In vitro expression of (A) herpes simplex virus type 1 thymidine kinase (HSV1-TK) or (B) Fms-like tyrosine kinase 3 ligand (Flt3L) in J3T cells infected with high-capacity adenovirus vectors (HC-Ads). (A) Diagram of the HC-Ad encoding HSV1-TK under the control of the murine cytomegalovirus (mCMV) promoter, indicating the different components and the orientation of the cassettes and their promoters. The mCMV promoter constitutively drives the expression of HSV1-TK. J3T dog glioma cells were infected with 1 transgene-expressing particle/cell of an HC-Ad encoding HSV1-TK (HC-Ad-TK) under the control of the mCMV promoter. After 48 h, transgene expression was determined by immunocytochemistry in the absence of ganciclovir (GCV). TK cytotoxicity was determined by the MTS viability assay in cells incubated for seven days in the presence of GCV (10 μM). Columns represent the mean ± SEM of percentage of cell viability, as determined by the MTS assay (n = 10 wells/group). (*p < 0.05 vs. GCV+ vs. GCV-; ^p < 0.05 vs. mock-infected cells.) Two-way analysis of variance (ANOVA) followed by Duncan's test. (B) Diagram of the HC-Ad encoding Flt3L- and the mCMV-driven regulatable tetracycline-dependent (TetON) cassette. The constructs indicate the individual components and the orientation of the cassettes and their promoters. The mCMV promoter drives the expression of the tetracycline-dependent transactivator (rtTA2SM2), which in the presence of doxycycline (DOX) activates the tetracycline-responsive element minimal (TRE) promoter to drive the expression of the Flt3L. J3T dog glioma cells were infected with 5,000 viral particles/cell of HC-Ad-mTetON-Flt3L for 72 h in the presence or absence of DOX (1 μg/ml). Transgene expression was determined by immunocytochemistry (right panels) and by ELISA in the cultures' supernatants. Columns represent the mean ± SEM of Flt3L concentration, as determined by ELISA (n = 3 wells/group). (*p < 0.05 vs. DOX+ vs. DOX-; ^p < 0.05 vs. mock-infected cells.) Two-way ANOVA followed by Duncan's test.
Combined therapy also exerted stringent control of Flt3L expression (Fig. 6B). In the absence of the inducer DOX, expression of Flt3L was negligible, whereas in the presence of DOX, combined therapy resulted in Flt3L levels of 4 nM, approximately 40% (p < 0.05) lower than the expression levels elicited by infection with HC-Ad-mTetON-Flt3L alone (Fig. 6B). However, transduction efficiency, as assessed by immunocytochemistry, was not significantly reduced when combined therapy was applied to J3T cells (approx. 50%).
In vitro expression of herpes simplex virus type 1 thymidine kinase (HSV1-TK) and Fms-like tyrosine kinase 3 ligand (Flt3L) in J3T cells infected with a combination of high-capacity adenovirus vectors (HC-Ads) expressing the therapeutic transgenes. J3T cells were infected with HC-Ad-TK and/or HC-mTetON-Flt3L in the presence or absence of doxycycline (DOX, 1 μg/ml). After 48 h, transgene expression was determined by immunocytochemistry and ELISA. TK cytotoxicity was determined by the MTS viability assay in cells incubated for an additional seven days with or without ganciclovir (GCV, 10 μM) and DOX. (A) Left panel: HSV1-TK cytotoxicity in J3T cells infected with HC-Ad-TK (1 transgene-expressing particle [TEP]/cell) alone or in combination with HC-Ad-mTetON-Flt3L (5,000 viral particles [VP]/cell) incubated in the presence or absence of GCV and DOX. Columns represent the mean ± SEM of percentage of cell viability (n = 10 wells/group). (*p < 0.05 vs. GCV+/DOX+ vs. GCV-/DOX-; ns, nonsignificant.) Two-way analysis of variance (ANOVA). Right panels: HSV1-TK expression in J3T cells infected with HC-Ad-TK alone or in combination with HC-Ad-mTetON-Flt3L. Results indicate percentage of transduced cells. (B) Left panel: Flt3L release was determined in the supernatant of J3T dog glioma cells infected with 5,000 VP/cell of HC-Ad-mTetON-Flt3L alone or in combination with HC-Ad-TK (1 TEP/cell) in the presence of DOX. Columns represent the mean ± SEM of Flt3L concentration as percentage of control (n = 3 wells/group). (*p < 0.05 vs. HC-Ad-mTetON-Flt3L.) Student's t-test. Right panels: Flt3L expression in J3T cells infected with HC-Ad-mTetON-Flt3L alone or in combination with HC-Ad-TK. Results depict percentage of transduced cells.
Discussion
In this article, we aimed to assess whether the mCMV promoter encoded within Ads was significantly more efficient both in the brain in vivo and in dog GBM cells in vitro at driving transgene expression. This would enable the use of lower vector doses with the concomitant decrease in any putative toxic side effects caused by higher vector loads, which would otherwise be required to achieve the necessary levels of therapeutic gene expression to elicit the desired clinical outcome.55 Our results tally with our previous reports in rat brain in vivo30,31 and human glioma cells in vitro.32 Our data also show that Ad-mCMV-β-Gal readily transduced astrocytes and neurons in vivo without inducing any local or systemic adverse side effects. Although transgene expression elicited by Ad-mCMV-β-Gal was higher and more widespread than by Ad-hCMV-β-Gal, the infiltration of inflammatory cells in the brain parenchyma at the site of Ad injection was similar for both vectors. The dose we used (8 × 107 PFU/injection site) was well tolerated and did not elicit short-term side effects other than slight local inflammation characterized by mononuclear pleocytosis evident in the CSF and the infiltration of macrophages and T cells in the vicinity of the injection site within the brain parenchyma. We have previously demonstrated the local and transient inflammatory response observed after Ad delivery into brain in vivo is dose dependent and does not adversely affect the levels or the duration of transgene expression.34-36,43 In the presence of preexposure to adenovirus, the adaptive immune response that later ensues eliminates transgene expression from first-generation Ads. This is because, even when the E1A genes that mediate transcription of all other Ad viral genes are deleted, there is still a very low-level leaky expression of Ad genes,56 which are recognized by cytotoxic anti-Ad immune cells.35,36,57
We also show that the mCMV promoter is more effective not only in the context of first-generation Ads, but also within regulatable HC-Ads. Although both regulatable HC-Ads (HC-Ad-mTetON-β-Gal and HC-Ad-hTetON-β-Gal) transduced the majority of dog glioma cells and exhibited stringent control by the inducer, β-Gal activity in the induced state was significantly higher when the TetON system was driven by the mCMV promoter. Our results suggest the mCMV promoter is an ideal promoter to achieve HC-Ad-mediated inducible therapeutic gene delivery to dog GBM, and that these constructs will enable the decrease in the overall dose of HC-Ad required to achieve a desirable therapeutic effect without compromising the safety of the therapy or its immune/inflammatory adverse side effects.
Preexisting immunity against gene-therapy vectors might not only hamper long-term transgene expression, but may also elicit very severe local and systemic adverse immune side effects.55 However, regulatable HC-Ads efficiently regulate transgene expression even in the presence of a systemic immune response against adenoviruses.31 We found that 60% of the outbred dogs tested exhibited neutralizing antibodies against adenoviruses. These data are in accordance with the anti-Ad immune status reported for humans.37 Thus, HC-Ads would be ideally suited for the treatment of GBM in dog and human patients. We assessed the ability of novel, therapeutic HC-Ads to infect and exert antitumoral effects in dog GBM cells in culture. We tested HC-Ads encoding HSV1-TK and Flt3L since overexpression of these two transgenes in combination with GCV showed effective antiglioma effects in a preclinical model of intracranial, syngeneic GBM in Lewis rats.15 Since we found that the mCMV promoter was more effective not only in the context of first-generation Ads, but also within regulatable HC-Ads, the therapeutic HC-Ads encoded TK and Flt3L under the control of the mCMV promoter. The expression of HC-Ad-TK and HC-Ad-mTetON-Flt3L resulted in effective transduction of dog glioma cells and exerted a strong cytotoxic effect when combined with GCV. Although HC-Ad-TK transduction efficiency was 25% as determined by immunocytochemistry, HSV1-TK cytotoxicity decreased the viability of dog glioma cells by 90%, suggesting the occurrence of a bystander effect of TK/GCV, as previously reported in other cell types.54 Moreover, a 15% decrease in cell viability in HC-Ad-TK-infected cells in the absence of GCV indicates that HSV1-TK is slightly cytotoxic for these cells, a phenomenon observed in other cell lines, including human glioma U251 cells.32,54,58
Transduction efficiency of HC-Ad-mTetON-Flt3L was approximately twofold higher when compared with HC-Ad-TK in dog GBM cells. This could be because Flt3L expression is driven by the TetON system, which yields higher levels of transgene expression in vivo and in vitro.31,32 Also, the tetracycline-controlled transcriptional silencer, tTSKid, not only allows for even tighter regulation of the inducible system,41 but also enhances transactivator expression levels by inhibiting its degradation.59
Since this therapy needs to be given in combination, we tested the transduction efficiency and bioactivity when the dog GBM cells were coinfected with both HC-Ads. Cytotoxicity induced by HC-Ad-TK in the presence of GCV was unmodified by coinfection. Although expression of one transgene did not block expression of the other one, coinfection resulted in lower levels of Flt3L being released. Since coinfection did not induce cell death per se when compared with HC-Ad-TK single infection, we conclude that this inhibitory effect was not caused by vector-induced cytotoxicity. More likely, since the two transgenes are driven by the same mCMV promoter, competition for the transcriptional and translational machinery in coinfected cells could result in lower expression levels of each individual transgene. Nevertheless, since Flt3L exerts an immune-stimulatory effect,15,47 and HSV1-TK exhibits an strong bystander effect,32,54,58 limited numbers of cells will need to be transduced for the therapy to be effective.
In summary, our results show for the first time that the mCMV promoter is stronger than the hCMV promoter in the normal brain of dogs in vivo and in dog glioma cells in vitro. Our data also demonstrate that HC-Ads encoding therapeutic transgenes under the control of regulatory sequences driven by the mCMV promoter elicit efficient therapeutic gene expression and cytotoxicity within canine GBM cells in vitro. These studies provide the foundations for the development of HC-Ads to implement gene-therapy trials in canines bearing spontaneous GBM. The results obtained in this large-animal model could be more predictive of therapeutic outcomes in human patients.
The work described in this article was funded by the National Institute of Neurological Disorders & Stroke (NINDS) grants 1RO1 NS44556.01 1RO3TW006273-01, 1R21 NSO54143.01, and 1UO1 NSO5246S.01 to M.G.C.; NINDS grants 1 RO1 NS42893, U54 4 NS04-5309, R21 NS47298, and 1RO1 NS054193.01 to P.R.L.; the Linda Tallen & David Paul Kane Annual Fellowship; the Bram and Elaine Goldsmith Endowed Chair to P.R.L.; the Medallions Group Endowed Chair to M.G.C.; and the Board of Governors at Cedars-Sinai Medical Center. M.C. is supported by NIH/NINDS 1F32 NS 058156.01.
References
Castro MG, Cowen R, Williamson IK, et al. Current and future strategies for the treatment of malignant brain tumors.
Curtin JF, King GD, Candolfi M, et al. Combining cytotoxic and immune-mediated gene therapy to treat brain tumors.
Gomez-Manzano C, Yung WK, Alemany R, Fueyo J. Genetically modified adenoviruses against gliomas: from bench to bedside.
King GD, Curtin JF, Candolfi M, Kroeger K, Lowenstein PR, Castro MG. Gene therapy and targeted toxins for glioma.
Prados MD, Levin V. Biology and treatment of malignant glioma.
Castro MG, Curtin J, King GD, et al. Novel gene therapeutic approaches to brain cancer. In: Castro MG, Lowenstein PR, eds.
Chiocca EA, Abbed KM, Tatter S, et al. A phase I open-label, dose-escalation, multi-institutional trial of injection with an E1B-attenuated adenovirus, ONYX-015, into the peritumoral region of recurrent malignant gliomas, in the adjuvant setting.
Fueyo J, Alemany R, Gomez-Manzano C, et al. Preclinical characterization of the antiglioma activity of a tropism-enhanced adenovirus targeted to the retinoblastoma pathway.
Germano IM, Fable J, Gultekin SH, Silvers A. Adenovirus/herpes simplex-thymidine kinase/ganciclovir complex: preliminary results of a phase I trial in patients with recurrent malignant gliomas.
Immonen A, Vapalahti M, Tyynela K, et al. AdvHSV-tk gene therapy with intravenous ganciclovir improves survival in human malignant glioma: a randomised, controlled study.
Lang FF, Bruner JM, Fuller GN, et al. Phase I trial of adenovirus-mediated p53 gene therapy for recurrent glioma: biological and clinical results.
Sandmair AM, Loimas S, Puranen P, et al. Thymidine kinase gene therapy for human malignant glioma, using replication-deficient retroviruses or adenoviruses.
Ali S, Curtin JF, Zirger JM, et al. Inflammatory and anti-glioma effects of an adenovirus expressing human soluble Fms-like tyrosine kinase 3 ligand (hsFlt3L): treatment with hsFlt3L inhibits intracranial glioma progression.
Ali S, King GD, Curtin JF, et al. Combined immunostimulation and conditional cytotoxic gene therapy provide long-term survival in a large glioma model.
Tyminski E, Leroy S, Terada K, et al. Brain tumor oncolysis with replication-conditional herpes simplex virus type 1 expressing the prodrug-activating genes, CYP2B1 and secreted human intestinal carboxylesterase, in combination with cyclophosphamide and irinotecan.
Herrlinger U, Jacobs A, Quinones A, et al. Helper virus-free herpes simplex virus type 1 amplicon vectors for granulocyte-macrophage colony-stimulating factor-enhanced vaccination therapy for experimental glioma.
Breen M. Development of molecular cytogenetic resources for the dog and application to the study of the comparative pathology of canine myeloid neoplasms. Paper presented at the
Braund K. Neoplasia of the nervous system. In: KG B, ed. Clinical Neurology in Small Animals: Localization, Diagnosis and Treatment. Ithaca, NY: International Veterinary Information Service; 2003. Available at http://www.ivis.org.
Foster ES, Carrillo JM, Patnaik AK. Clinical signs of tumors affecting the rostral cerebrum in 43 dogs.
Heidner GL, Kornegay JN, Page RL, Dodge RK, Thrall DE. Analysis of survival in a retrospective study of 86 dogs with brain tumors.
LeCouteur RA. Current concepts in the diagnosis and treatment of brain tumours in dogs and cats.
Lipsitz D, Higgins RJ, Kortz GD, et al. Glioblastoma multiforme: clinical findings, magnetic resonance imaging, and pathology in five dogs.
Lindblad-Toh K, Wade CM, Mikkelsen TS, et al. Genome sequence, comparative analysis and haplotype structure of the domestic dog.
Cerletti M, Negri T, Cozzi F, et al. Dystrophic phenotype of canine X-linked muscular dystrophy is mitigated by adenovirus-mediated utrophin gene transfer.
Childers MK, Okamura CS, Bogan DJ, Bogan JR, Sullivan MJ, Kornegay JN. Myofiber injury and regeneration in a canine homologue of Duchenne muscular dystrophy.
High K. Gene transfer for hemophilia: can therapeutic efficacy in large animals be safely translated to patients?
Ponder KP, Melniczek JR, Xu L, et al. Therapeutic neonatal hepatic gene therapy in mucopolysaccharidosis VII dogs.
Wood PA, Sisk DB, Styer E, Baker HJ. Animal model: ceroidosis (ceroidlipofuscinosis) in Australian cattle dogs.
Gerdes CA, Castro MG, Lowenstein PR. Strong promoters are the key to highly efficient, noninflammatory and noncytotoxic adenoviral-mediated transgene delivery into the brain in vivo.
Xiong W, Goverdhana S, Sciascia SA, et al. Regulatable gutless adenovirus vectors sustain inducible transgene expression in the brain in the presence of an immune response against adenoviruses.
Candolfi M, Curtin JF, Xiong W, et al. Effective high capacity gutless adenoviral vectors mediate transgene expression in human glioma cells.
Lowenstein PR, Thomas CE, Umana P, et al. High-capacity, helper-dependent, “gutless” adenoviral vectors for gene transfer into the brain.
Lowenstein PR. Virology and immunology of gene therapy, or virology and immunology of high MOI infection with defective viruses.
Thomas CE, Schiedner G, Kochanek S, Castro MG, Lowenstein PR. Peripheral infection with adenovirus causes unexpected long-term brain inflammation in animals injected intracranially with first-generation, but not with high-capacity, adenovirus vectors: toward realistic long-term neurological gene therapy for chronic diseases.
Thomas CE, Schiedner G, Kochanek S, Castro MG, Lowenstein PR. Preexisting antiadenoviral immunity is not a barrier to efficient and stable transduction of the brain, mediated by novel high-capacity adenovirus vectors.
Chirmule N, Propert K, Magosin S, Qian Y, Qian R, Wilson J. Immune responses to adenovirus and adeno-associated virus in humans.
Ehrhardt A, Kay MA. Gutted adenovirus: a rising star on the horizon?
Southgate T, Kingston P, Castro MG. Gene transfer into neural cells in vivo using adenoviral vectors. In: Gerfen CR, McKay R, Rogawski MA, Sibley DR, Skolnick P, eds.
Umana P, Gerdes CA, Stone D, et al. Efficient FLPe recombinase enables scalable production of helper-dependent adenoviral vectors with negligible helper-virus contamination.
Goverdhana S, Puntel M, Xiong W, et al. Regulatable gene expression systems for gene therapy applications: progress and future challenges.
Dewey RA, Morrissey G, Cowsill CM, et al. Chronic brain inflammation and persistent herpes simplex virus 1 thymidine kinase expression in survivors of syngeneic glioma treated by adenovirus-mediated gene therapy: implications for clinical trials.
Thomas CE, Birkett D, Anozie I, Castro MG, Lowenstein PR. Acute direct adenoviral vector cytotoxicity and chronic, but not acute, inflammatory responses correlate with decreased vector-mediated transgene expression in the brain.
Candolfi M, Kroeger KM, Pluhar GE, et al. Adenoviral-mediated gene transfer into the canine brain in vivo.
Rainov NG, Koch S, Sena-Esteves M, Berens ME. Characterization of a canine glioma cell line as related to established experimental brain tumor models.
Smith-Arica JR, Morelli AE, Larregina AT, Smith J, Lowenstein PR, Castro MG. Cell-type-specific and regulatable transgenesis in the adult brain: adenovirus-encoded combined transcriptional targeting and inducible transgene expression.
Curtin JF, King GD, Barcia C, et al. Fms-like tyrosine kinase 3 ligand recruits plasmacytoid dendritic cells to the brain.
Stein VM, Czub M, Schreiner N, et al. Microglial cell activation in demyelinating canine distemper lesions.
Zirger JM, Liu C, Barcia C, Castro MG, Lowenstein PR. Immune regulation of transgene expression in the brain: B cells regulate an early phase of elimination of transgene expression from adenoviral vectors.
Lowenstein PR, Castro MG. Progress and challenges in viral vector-mediated gene transfer to the brain.
Zirger JM, Barcia C, Liu C, et al. Rapid upregulation of interferon-regulated and chemokine mRNAs upon injection of 108 international units, but not lower doses, of adenoviral vectors into the brain.
Palmer D, Ng P. Improved system for helper-dependent adenoviral vector production.
Palmer DJ, Ng P. Physical and infectious titers of helper-dependent adenoviral vectors: a method of direct comparison to the adenovirus reference material.
Maleniak TC, Darling JL, Lowenstein PR, Castro MG. Adenovirus-mediated expression of HSV1-TK or Fas ligand induces cell death in primary human glioma-derived cell cultures that are resistant to the chemotherapeutic agent CCNU.
Raper SE, Chirmule N, Lee FS, et al. Fatal systemic inflammatory response syndrome in a ornithine transcarbamylase deficient patient following adenoviral gene transfer.
Yang Y, Nunes FA, Berencsi K, Furth EE, Gonczol E, Wilson JM. Cellular immunity to viral antigens limits E1-deleted adenoviruses for gene therapy.
Barcia C, Thomas CE, Curtin JF, et al. In vivo mature immunological synapses forming SMACs mediate clearance of virally infected astrocytes from the brain.
Windeatt S, Southgate TD, Dewey RA, et al. Adenovirus-mediated herpes simplex virus type-1 thymidine kinase gene therapy suppresses oestrogen-induced pituitary prolactinomas.



![In vitro expression of herpes simplex virus type 1 thymidine kinase (HSV1-TK) and Fms-like tyrosine kinase 3 ligand (Flt3L) in J3T cells infected with a combination of high-capacity adenovirus vectors (HC-Ads) expressing the therapeutic transgenes. J3T cells were infected with HC-Ad-TK and/or HC-mTetON-Flt3L in the presence or absence of doxycycline (DOX, 1 μg/ml). After 48 h, transgene expression was determined by immunocytochemistry and ELISA. TK cytotoxicity was determined by the MTS viability assay in cells incubated for an additional seven days with or without ganciclovir (GCV, 10 μM) and DOX. (A) Left panel: HSV1-TK cytotoxicity in J3T cells infected with HC-Ad-TK (1 transgene-expressing particle [TEP]/cell) alone or in combination with HC-Ad-mTetON-Flt3L (5,000 viral particles [VP]/cell) incubated in the presence or absence of GCV and DOX. Columns represent the mean ± SEM of percentage of cell viability (n = 10 wells/group). (*p < 0.05 vs. GCV+/DOX+ vs. GCV-/DOX-; ns, nonsignificant.) Two-way analysis of variance (ANOVA). Right panels: HSV1-TK expression in J3T cells infected with HC-Ad-TK alone or in combination with HC-Ad-mTetON-Flt3L. Results indicate percentage of transduced cells. (B) Left panel: Flt3L release was determined in the supernatant of J3T dog glioma cells infected with 5,000 VP/cell of HC-Ad-mTetON-Flt3L alone or in combination with HC-Ad-TK (1 TEP/cell) in the presence of DOX. Columns represent the mean ± SEM of Flt3L concentration as percentage of control (n = 3 wells/group). (*p < 0.05 vs. HC-Ad-mTetON-Flt3L.) Student's t-test. Right panels: Flt3L expression in J3T cells infected with HC-Ad-mTetON-Flt3L alone or in combination with HC-Ad-TK. Results depict percentage of transduced cells.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/neuro-oncology/9/3/10.1215_15228517-2007-012/1/m_Fig_06.gif?Expires=1716901710&Signature=hGQPIJHu-fY26PoB-llDV2OaprYp9Uj1aHW6EZXij3oUalVV4BTrgcaFJUIXt8Xkkp0et6jXfncLSMwLgXXpublLP9nMf23dUI6rMYaHelolGhlif5GAtX66vzBR9jnnvTrBtfDeEx3e~mr1Dh-VGUZwCHSs0UNbYocCoR1VEIh24VoUKEfEMlfXiITtgOM1Msw2AKrhn151cHop-pSxzpQc6aMtyEvgtSIiiINdI73MgIqrqVoBoBunvDiQTw9SfxZJHTyHq~XUJqjTdnMGp4gfyRGGP~~M2xGIbpwbWpdnBwAjZn6t-woGxeUxmBnYsgdaB8dnYUZzqSz4g904Iw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

