1
Max Planck Institute for Human Development, Berlin, Germany
2
Berlin Neuroimaging Center, Berlin, Germany
3
Max Delbrück Center for Molecular Medicine, Berlin, Germany
4
Department of Neurology, Charité University Medicine, Berlin, Germany
5
Max Planck Institute for Human Cognitive and Brain Sciences, Leipzig, Germany
6
Aging Research Center, Karolinska Institute, Stockholm, Sweden
We demonstrate that common genetic polymorphisms contribute to the increasing heterogeneity of cognitive functioning in old age. We assess two common Val/Met polymorphisms, one affecting the Catechol-O-Methyltransferase (COMT) enzyme, which degrades dopamine (DA) in prefrontal cortex (PFC), and the other influencing the brain-derived neurotrophic factor (BDNF) protein. In two tasks (Wisconsin Card Sorting and spatial working memory), we find that effects of COMT genotype on cognitive performance are magnified in old age and modulated by BDNF genotype. Older COMT Val homozygotes showed particularly low levels of performance if they were also BDNF Met carriers. The age-associated magnification of COMT gene effects provides novel information on the inverted U-shaped relation linking dopaminergic neuromodulation in PFC to cognitive performance. The modulation of COMT effects by BDNF extends recent evidence of close interactions between frontal and medial-temporal circuitries in executive functioning and working memory.
Individual differences in complex phenotypes result from gene-gene and gene-context interactions (McClearn, 2006
). Between-person variation in brain functions and cognitive abilities is associated with genetic factors (Klein et al., 2007
; Meyer-Lindenberg et al., 2007
; Savitz et al., 2006
), and changes across the lifespan (Baltes et al., 1999
; Cabeza et al., 2005
; Diamond, 2007
; Diamond et al., 2004
; Posner et al., 2007
). Recently, the Catechol-O-Methyltransferase (COMT) gene, implicated in executive functions, and the Brain-Derived Neurotrophic Factor (BDNF) gene, associated with memory-related functions, have received increasing attention in gene-cognition association studies (Goldberg and Weinberger, 2004
; Savitz et al., 2006
). Here, we examine whether normal human aging magnifies the effects of these genes on executive functioning and working memory.
COMT enzymatic activity results in degradation of dopamine (DA) and thus has an impact on endogenous DA levels in prefrontal cortex (PFC). A common polymorphism of the COMT gene is associated with most of the human variation in intrinsic DA levels in the PFC. The COMT single nucleotide polymorphism leads to a substitution of valine (Val) with methionine (Met) at the codon 158 on chromosome 22q11 (Val158Met). This substitution affects enzymatic activity, which is three to four times higher in Val than in Met homozygotes. Lower enzymatic activity among Met carriers leads to less frontal DA degradation and hence greater DA availability at the receptors (Meyer-Lindenberg et al., 2007
).
Some behavioral studies using tasks that tax executive functioning such as the Wisconsin Card Sorting Test (WCST) and n-back task have found an advantage of Met over Val carriers in young adults. However, effect sizes are generally small (Egan et al., 2001
; Malhotra et al., 2002
; Meyer-Lindenberg et al., 2006
) and not always statistically reliable (Barnett et al., 2007
). One reason for the relatively small effects may be that the advantage of Met carriers in executively demanding tasks applies to sustained processes such as maintaining a cognitive set, but not to transient processes related to cognitive flexibility (Bilder et al., 2004
; Grace et al., 2007
).
Animal and human data suggest that the relation between DA levels and cognitive functioning follows an inverted U-shaped function (Goldman-Rakic et al., 2000
; Li and Sikström, 2002
; Li et al., 2001
; Mattay et al., 2003
; Vijayraghavan et al., 2007
). The DA system undergoes a marked decline with increasing adult age, with a gradual loss of both pre- and post-synaptic markers of DA neurotransmission from early through late adulthood (Antonini and Leenders, 1993
; Erixon-Lindroth et al., 2005
; Kaasinen et al., 2000
; Suhara et al., 1991
). This loss is consistently found in striatal, neocortical (e.g., frontal), and limbic areas (Bäckman et al., 2006
). Normal human aging is also associated with decline across a range of cognitive abilities (Baltes and Lindenberger, 1997
). Higher-order cognitive functions that rely on PFC and medial-temporal lobe (MTL) integrity (e.g., working memory, executive functions) show a particularly robust age-related decline (Bäckman et al., 1999
; Raz et al., 2007
; West, 1996
). Further, molecular imaging studies indicate that age-related DA losses are powerful mediators of age-related impairment in multiple cognitive tasks, including those assessing working memory and executive functions (Bäckman et al., 2000
, 2006
; Erixon-Lindroth et al., 2005
; Volkow et al., 1998
).
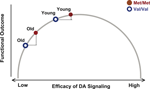
Given the close association between deficient dopaminergic neuromodulation and age-related cognitive decline (Bäckman et al., 2006
), it is plausible to assume that advancing adult age shifts individuals toward the left-hand side of the curve relating DA signaling to cognitive performance, that is, further and further away from the functional optimum. The deleterious effects of this leftward shift should be particularly pronounced among individuals with relatively low DA levels in young adulthood, such as Val carriers of the COMT gene. Thus, we predict that human aging magnifies the effects of the COMT polymorphism on cognitive performance (Figure 1
). Thus far, most studies investigating COMT effects on cognitive performance have involved younger adults. However, in line with our prediction, the few studies with older adults consistently report a clear advantage of Met over Val carriers on cognitive tasks assessing working memory and related functions (de Frias et al., 2004
, 2005
; Harris et al., 2005
; Mattay et al., 2006
; Starr et al., 2007
).
Figure 1. Inverted U-shaped function linking the efficacy of frontal DA signaling in early versus late adulthood to performance. The shape of the curve implies that the difference in performance between older Met and Val carriers is greater than the difference between younger Met and Val carriers of the COMT gene.
Recent association studies stress the need to consider gene-gene interactions (Kovas and Plomin, 2006
; Tan et al., 2007
; Yacubian et al., 2007
). Studies on gene-gene interactions involving COMT have focused on interdependencies between the COMT gene and other genes linked to the catecholaminergic system, such as the DA transporter gene (Yacubian et al., 2007
) and the serotonin-related 5-HTTLPR gene (Smolka et al., 2007
). Here, we focus on the interaction between the COMT gene and the common Val/Met polymorphism affecting the BDNF protein, which enhances MTL-related mechanisms such as long-term potentiation and binding (Egan et al., 2003
). Secretion of BDNF is higher in Val homozygotes than in Met carriers. BDNF Val homozygotes have greater hippocampal volume and task-related brain activation as well as higher performance in memory tasks than Met carriers (Egan et al., 2003
; Hariri et al., 2003
; Pezawas et al., 2004
).
The influence of BDNF on brain and cognition is not restricted to MTL-dependent memory processes. Met carriers show reduced grey-matter volume in prefrontal cortex (Xu et al., 2007
), and perform less well on the WCST (Rybakowski et al., 2003
). Furthermore, BDNF influences DA release in striatal regions (Narita et al., 2003
), which may interact with COMT effects on prefrontal DA catabolism through basal ganglia-thalamocortical loops (Alexander et al., 1986
). Thus, for several reasons, the COMT and BDNF genes may interactively influence executive functioning and working memory. First, PFC and MTL are strongly connected and co-activated during a wide range of executive tasks (Cabeza et al., 2003
; Courtney et al., 1997
; Rypma and D’Esposito, 2000
). Second, although COMT has primarily been associated with PFC (Meyer-Lindenberg et al., 2007
), and BDNF with MTL (Hariri et al., 2003
), both genes influence both brain regions (Bertolino et al., 2006
; Pezawas et al., 2004
). Therefore, we also examined whether age differences in the effects of the COMT Val/Met polymorphism on executive functioning and working memory are modulated by the Val/Met BDNF polymorphism.
In sum, our key prediction was that human aging magnifies the functional consequences of genetic variations affecting DA signaling (Figure 1
). This prediction is derived from the inverted U-shaped relation between DA signaling and cognitive performance, and the important role of deficient dopaminergic neuromodulation in cognitive aging. Furthermore, given the role of BDNF in frontal integrity (Pezawas et al., 2004
) and the role of the MTL in executive functioning (Courtney et al., 1997
; Takahashi et al., 2007
), we also predicted that the relation between COMT status and adult age is modulated by BDNF genotype. We expected that older COMT Val carriers who also carry the Met allele of the BDNF gene would show particularly low levels of executive performance. To test these predictions, younger and older adults were tested on the WCST and on a spatial working memory (SWM) task. We chose a SWM task because spatial processing is known to be particularly sensitive to aging (Jenkins et al., 2000
) as well as to dopamine status (Bäckman et al., 2006
).
Participants
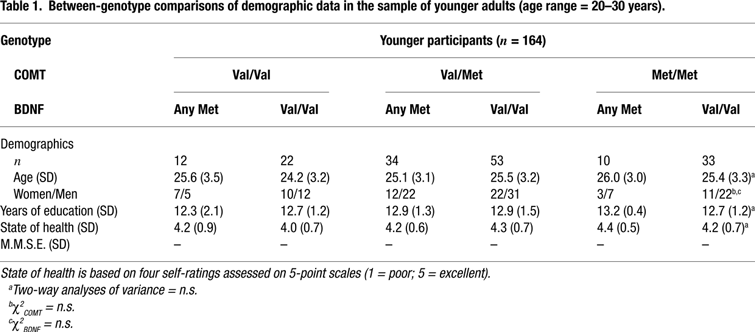
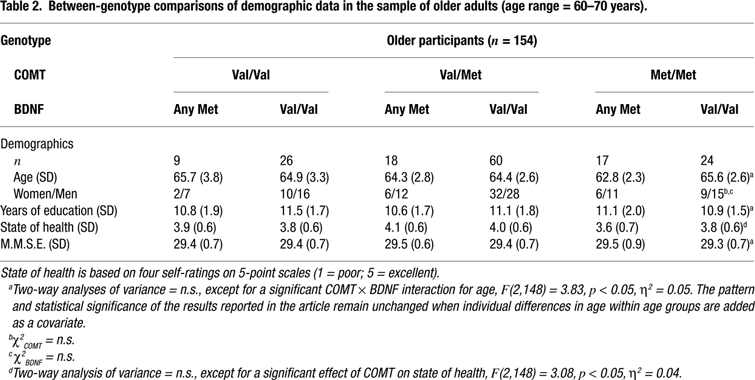
We studied 318 healthy volunteers from two age groups: 164 younger adults (age range = 20–31, mean age = 25, SD = 3.2, 65 women and 99 men) and 154 older adults (age range = 60–70, mean age = 65, SD = 2.9, 65 women and 89 men). All subjects were right-hand dominant and had normal or corrected vision. The older sample had no symptoms of dementia (all scores on the Mini-Mental Status Examination were over 27 out of 30) and none of them reported a history of medical, neurological, or psychiatric disease. Subjects were categorized according to their allelic variants of the COMT and BDNF polymorphisms. They were recruited via newspaper announcements, posters in public transportation, and postcards. Subjects gave informed consent and were paid for their participation. The study was approved by the ethics committees of the Max Planck Institute for Human Development and the Charité University Medicine, Berlin. For detailed sample description, see Tables 1 and 2
.
Genotyping
DNA was extracted from peripheral blood using standard methods. The non-synonymous mutation of rs4680 of the COMT Val158Met polymorphism as well as the rs6265 BDNF Val66Met polymorphism were genotyped in a 384-well microtiter plate format using the TaqMan 5quotidn-exonuclease assay as described elsewhere (Egan et al., 2001
; Hariri et al., 2003
). Both SNPs were selected from the dbSNP (http://www.ncbi.nlm.nih.gov/SNP/). The sequences of primers and TaqMan probes for the SNP genotyping were designed and synthesized by Applied Biosystems (Foster City, CA, USA).
The frequencies of the three COMT genotypes were 0.21 for Val/Val, 0.53 for Val/Met and 0.26 for met/met for the younger subjects and 0.23 for Val/Val, 0.50 for Val/Met and 0.27 for Met/Met for the older subjects. The frequencies of the two BDNF genotypes were 0.34 for any Met and 0.66 for Val/Val for the younger subjects and 0.29 for any Met and 0.71 for Val/Val for the older subjects. The allelic distributions of the each gene did not deviate significantly from those expected according to Hardy-Weinberg equilibrium in the younger, COMT: χ2(1) = 0.68, p > 0.10; BDNF: χ2(1) = 0.01, p > 0.10, or the older subjects, COMT: χ2(1) = 0.03, p > 0.10; BDNF: χ2(1) = 1.43, p > 0.10. The observed frequencies did not differ between the two age groups, COMT: χ2(2) = 0.24, p > 0.10; BDNF: χ2(1) = 1.15, p > 0.10. After genotype determination, the groups were divided based on COMT-BDNF genotypes. Demographic and clinical data are given in Tables 1 and 2
.
Cognitive Tasks
Participants underwent two cognitive testing sessions, lasting approximately 2.5 h each. These sessions were held in groups of about six subjects of the same age. The cognitive tasks were administered to investigate executive functioning, episodic memory, verbal ability, processing speed, and robustness. Two tasks (i.e., WCST and SWM) were used in the present study to assess executive functioning.
Wisconsin card sorting test. A computer-administered adapted version of the standard 128-cards WCST was used (Heaton et al., 1993
). This task is considered as a standard neuropsychological index of executive functioning. Four key cards are presented at the top of the screen. A response card is shown at the bottom center of the screen and has to be sorted by the participant to one of the cards presented on the top of the screen. The cards can be matched based on three dimensions: color, form, and number. Subjects respond by pressing one of the four corresponding buttons with the index finger of the right hand. A limited time is given to allow participants to correct their answer. Then, feedback about the correctness of the response appears briefly on the screen. Participants must use this feedback to sort the next response card as no explicit information about the current sorting rule or the switch to a new sorting rule is provided. The first sorting principle is color, followed by form and then number. When a person attains 10 correct consecutive sorting orders (referred to as completing a category), the sorting principle changes (in the order noted). This sequence is repeated once. Because no warning is given about these changes, subjects must make the necessary shift of mental set on the basis of the feedback given. The test continues until six categories have been completed or until the entire set of 128 cards has been sorted. Participants were instructed to perform as fast and accurately as possible. Performance was evaluated by applying the WCST standard scoring rules as described by (Heaton et al., 1993
). The percentage of perseverative errors and reaction times for correct responses were used to index performance.
Spatial working memory. We modified a computerized SWM task devised by Klingberg and colleagues (1997). In this task, participants were visually presented with a series of dots, displayed consecutively in a specific location in a 4 × 4 grid of circles. They had to decide if a dot was presented on the position of a specific circle (i.e. location memory condition). If subjects responded “yes” to the spatial location, a digit was presented in this position. Subjects then had to decide if the digit matched the serial position of the dot in the presented order (i.e. sequence memory condition). They responded with a right index-button press if the location or the serial position was correct, and with a left index-button press if the location or serial position was wrong. Load level was manipulated by the number of dots in the sequence; either four or seven (i.e., set size 4 and set size 7 conditions, respectively). One third of the items were associated with correct location and temporal order, one third with correct location and incorrect order, and another third with incorrect location (and, by implication, missing order information). The timing of the sequence was set up with a 1000 ms fixation, immediately followed by the stimulus presentation (600 ms per dot) and a marked circle for a maximum duration of 5000 ms. When serial order was assessed, a digit was presented in the same position for a maximum of 5000 ms. The interstimulus interval was 400 ms. The task involved 4 blocks of 24 trials each, each load level (i.e. size 4 or 7) being repeated twice, for a total of 48 trials per condition. The different load levels were presented in counterbalanced order (4-7-7-4), which was kept constant across participants. Reaction time and accuracy were recorded for each trial separately.
Statistical Analysis
Demographic data were analyzed with analyses of variance and χ2 tests, using SPSS for Windows 15.0 (SPSS, Chicago, IL). Behavioral data were analyzed with mixed-effect models with maximum-likelihood estimation (‘Proc Mixed’ procedure), using SAS 9.1 for Windows (SAS Institute Inc., Cary, NC, USA). In contrast to standard ANOVA, mixed-effect models allow for differences in variances and covariances between groups. Variances and covariances were allowed to differ between age groups because older adults were reliably more variable than younger adults. Allowing for unequal variances and covariances in the presence of variance-covariance heterogeneity permits unbiased tests of statistical significance, whereas standard ANOVA procedures would yield biased results. Here, the bias of standard ANOVA was progressive because the critical triple interaction originated from a subsample that was more variable than average. Thus, all effects reported here were also statistically reliable when standard ANOVA methods were used. The alpha level was set to p = 0.01, and exact p values are reported if 0.01 < p < 0.05. Effect sizes are indicated by the intraclass correlation coefficient (ρI). The ρI values were squared to ease interpretation in terms of the percentage of total variance associated with an effect.
Wisconsin Card Sorting Test (WCST)
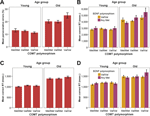
Percent perseverative errors and reaction times for correct responses on the WCST are presented in Figures 2
A,B. For both measures, 3-way ANOVAs were conducted to examine the associations among age (young, old), COMT genotype (Val/Val, Val/Met, Met/Met), and BDNF genotype (Val/Val, any Met), using sex as a covariate. Older participants committed more perseverative errors than younger participants, F(1, 256) = 77.76, p < 0.01, ρI = 0.48, explaining 23.3% of the total variance in the data. There was no main effect of COMT genotype, F(2, 255) = 1.36, n.s., but the interaction between age and COMT was reliable, F(2, 256) = 3.01, p = 0.05, ρI = 0.15 (2.3%), reflecting a Val disadvantage for older adults only (for older Val versus older Met homozygotes, t = –2.22, p = 0.03; see Figure 2
A).
Figure 2. (A) Mean perseverative errors in the WCST as a function of age and COMT genotype. (B) Mean reaction time for correct WCST responses as a function of age, COMT genotype, and BDNF genotype. (C) Mean reaction time for correct SWM responses as a function of age and COMT genotype at load 4. (D) Mean reaction time for correct SWM responses as a function of age, COMT genotype, and BDNF genotype at load 7. Error bars represent standard errors around the means. WCST = Wisconsin Card Sorting Test; SWM = spatial working memory.
With respect to reaction times for correct responses, older adults responded more slowly than younger adults, F(1, 215) = 159.06, p < 0.01, ρI = 0.65 (42.5%). The effect of COMT genotype, F(2, 214) = 4.18, p = 0.02, ρI = 0.19 (3.8%), and the age × COMT interaction, F(2, 214) = 4.54, p = 0.01, ρI = 0.20 (4.1%), were significant. This interaction was qualified by a significant triple interaction of age, COMT, and BDNF, F(2, 214) = 3.43, p = 0.03, ρI = 0.18 (3.1%). Older homozygotic Val carriers of the COMT gene with at least one BDNF met allele were particularly slow (compared to the five other groups of older adults, t = –2.82, p < 0.01; see Figure 2
B).
Spatial Working Memory (SWM) Task
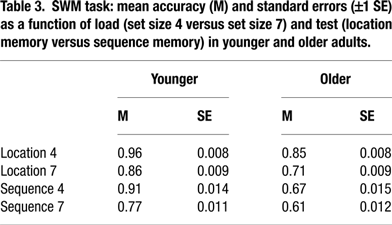
Performance accuracy in the SWM task was examined in a 5-way mixed ANOVA with age (young, old), COMT (Val/Val, Val/Met, Met/Met), and BDNF (Val/Val, any Met) as between-subject factors, load (4 items, 7 items) and test (identity, sequence) as within-subject factors, and sex as a covariate. No significant main effects or interactions involving either polymorphism were found, F(2, 239) = 1.41, n.s., for COMT; F(1, 240) = 2.11, n.s., for BDNF; and p > 0.05 for all interactions involving COMT or BDNF. For details on age, load, and test effects see Table 3
.
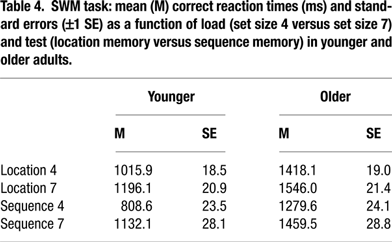
The corresponding 5-way mixed ANOVA on reaction times for correct responses revealed a significant 4-way interaction of age, COMT, BDNF, and load, F(2, 269) = 3.71, p = 0.02, ρI = 0.16 (2.7%), (for other effects of the 5-way ANOVA, see Table 4
).
To trace the source of the 4-way interaction, separate analyses for each load level were conducted. At set size 4, we found main effects of age, F(1, 256) = 288.97, p < 0.01, ρI = 0.73 (53.0%), test, F(1, 222) = 106.76, p < 0.01, ρI = 0.57 (32.5%), and an interaction between age and test, F(1, 222) = 3.98, p = 0.05, ρI = 0.13 (1.8%). Critically, the interaction between age and COMT was reliable, F(2, 255) = 3.29, p = 0.04, ρI = 0.16 (2.5%), indicating that slower responding among homozygotic Val carriers was restricted to the old (for older Val versus older Met homozygotes, t = –2.16, p = 0.03; Figure 2
C; see also Table 4
).
At set size 7, we found significant main effects of age, F(1, 261) = 131.69, p < 0.01, ρI = 0.58 (33.5%) and test, F(1, 239) = 14.20, p < 0.01, ρI = 0.24 (5.6%). None of the two-way interactions were statistically reliable, all ps > 0.10. Here, the triple interaction among age, COMT, and BDNF was significant, F(2, 259) =3.44, p = 0.03, ρI = 0.16 (2.6%). Older Val/Val COMT individuals who carry BDNF Met genes showed particularly elevated response times (compared to the five other groups of older adults, t = –2.55, p = 0.01; Figure 2
D).
We hypothesized that (a) human aging magnifies the influence of the Val/Met COMT polymorphism on executive functioning and working memory, and (b) this effect is modulated by the Val/Met BDNF polymorphism. Data obtained on two tasks were consistent with both predictions. First, for a standard measure of executive functioning, the WCST, the COMT Val allele was associated with a higher number of perseverative errors in older adults. This association was not observed in younger adults. In addition, reaction times for correct responses were also dependent on BDNF status: Older adults carrying two COMT Val alleles and at least one BDNF Met allele took particularly long time to respond, resulting in an age x gene x gene interaction.
Second, for the SWM task, genetic modulation of performance was restricted to reaction times for correct responses. In the low-load condition (set size 4), COMT effects on processing efficiency were not reliable in younger adults but present in older adults. This age x gene interaction pattern closely resembled the pattern found for perseverative errors in the WCST (cf. Figures 2
A,C). In the high-load condition (set size 7), we again found a triple interaction of age, COMT, and BDNF. Response times were particularly slow in older adults carrying two COMT Val alleles and at least one BDNF Met allele. This pattern mirrors the WCST latency data for correct responses (cf. Figures 2
B,D). The results from both tasks support the notion that effects of the Val/Met COMT polymorphism on executive functioning and working memory increase in old age, and are further accentuated by the corresponding BDNF polymorphism. Note that the genetic effects were stronger for latency than for accuracy, likely reflecting greater sensitivity of the former measure. However, the interactive effects of age, COMT, and BDNF reported here did not generalize to other speeded tasks in our cognitive battery such as simple reaction time. Thus, the present interactive effects are not a byproduct of age-differential genetic effects on response speed per se, but rather reflect demands on executive or working memory functioning.
Most investigations on cognitive effects of the COMT gene have included younger adults (Bilder et al., 2004
; Egan et al., 2001
; Ho et al., 2005
; Mattay et al., 2003
; Montague et al., 2004
). It may seem surprising that we did not observe reliable COMT effects in younger adults, as such effects have been observed in earlier studies with smaller samples (Egan et al., 2001
; Mattay et al., 2003
; Montague et al., 2004
). However, the evidence on COMT effects on executive functioning and working memory in early adulthood is not unequivocal, as several studies have failed to find such effects (Barnett et al., 2007
; Bilder et al., 2004
; Ho et al., 2005
; Tsai et al., 2003
). In contrast, the few cognitive studies with older adults have invariably reported COMT effects in the expected direction (de Frias et al., 2004
, 2005
; Harris et al., 2005
; Mattay et al., 2006
; Starr et al., 2007
). The direct age-comparative evidence obtained in this study provides novel support for the view that COMT effects are unmasked in old age, presumably because older adults are operating at suboptimal levels of dopaminergic neuromodulation.
Based on animal research, molecular imaging studies in humans, and neurocomputational modeling, we assumed that age-related declines in DA signaling lead to noisier and less efficient processing in PFC (Bäckman et al., 2006
; Li et al., 2001
). DA signaling is known to affect PFC-related cognitive functions in an inverted U-shaped manner (Goldman-Rakic et al., 2000
; Li and Sikström, 2002
; Mattay et al., 2003
; Vijayraghavan et al., 2007
). We also assumed that normal aging moves individuals further to the left on the inverted U-shaped curve, and that Val COMT carriers start this lifespan shift further to the left than Met COMT carriers. As a result, it follows that Val COMT carriers undergo a greater loss in DA signaling than Met carriers (Figure 1
). Our results are in excellent agreement with this model, thereby extending the validity of the inverted-U shaped function to normal human aging.
Past research on alleles of the COMT gene and cognition has yielded mixed results regarding the locus of the effect. Some research has reported that the key difference lies between homozygotic Met carriers and the remaining groups, others have found that the difference is confined to homozygotic Val carriers, and still others have documented dose-response effects (Bäckman et al., 2006
). Toward this end, it is of note that impaired performance in this study was restricted to old adults with two Val alleles of the COMT gene.
We also found that the interaction between age and COMT status was modulated by the BDNF gene. There is evidence that carriers of the BDNF Met allele perform more poorly in tasks assessing memory and fluid intelligence (Egan et al., 2003
; Hariri et al., 2003
; Ho et al., 2006
; Tsai et al., 2004
). Here, we found that older Met BDNF carriers took more time to respond in the WSCT and in the high-load condition of the SWM task if they were Val homozygotes on the COMT gene. The resulting triple interaction among age, COMT, and BDNF, in which BDNF further exacerbated the processing disadvantage of older individuals who are Val homozygotes on COMT, lends further support to our view that COMT and BDNF are jointly involved in regulating the PFC - MTL executive control - episodic memory circuitry (Buckner et al., 1996
; Miller and Cohen, 2001
). The influence of BDNF on this circuitry may have at least three origins. Anatomically, BDNF affects both PFC and hippocampal gray-matter volumes (Pezawas et al., 2004
). Neurochemically, BDNF influences DA release in striatal regions (Narita et al., 2003
) that may affect PFC functions through the striato-thalamo-cortical pathway (Alexander et al., 1986
). Functionally, the MTL and PFC jointly contribute to executive functioning, working memory, and episodic memory circuitries (Naghavi and Nyberg, 2005
). For instance, a recent study showed that DA D2 receptor binding efficacy in MTL is strongly related to WCST performance (Takahashi et al., 2007
). Similarly, patient studies show that various forms of MTL damage affect working memory in addition to episodic memory (Olson et al., 2006
; Piekema et al., 2007
).
Note that the BDNF gene - related effects on executive functioning and working memory were confined to old COMT Val/Val carriers (Figure 2
). In the other age and COMT groupings, the effect of the BDNF gene was negligible. In view of the robust effects of the BDNF gene on episodic memory (Egan et al., 2003
; Hariri et al., 2003
), the restricted influence in the present study suggests that effects of the BDNF gene are more salient in episodic memory than in executive functioning. Supportive evidence for this claim comes from a recent study contrasting the impact of this gene on a range of cognitive tasks (Ho et al., 2006
). Possibly, being a Met BDNF carrier is particularly detrimental for individuals with poor PFC functioning (i.e., older Val COMT homozygotes), because their executive functions are more dependent upon MTL-based binding mechanisms.
Given the present effects of the BDNF gene, it is important to note that a recent study with an elderly sample found that BDNF Met homozygotes scored higher than BDNF Val carriers on a test of fluid ability (Harris et al., 2006
). The discrepant findings may reflect sample differences. A protective effect of the BDNF Met allele for Alzheimer’s disease has been reported (Matsushita et al., 2005
; Ventriglia et al., 2002
). The older adults studied by Harris et al. (2006)
originated from a population-based sample. It is conceivable that their sample comprised a larger proportion of individuals at risk for dementia than the more select volunteer sample investigated here.
Longitudinal studies on adult development have shown that individual differences in cognitive performance are remarkably stable, even in the presence of sizeable mean-level decline (Deary et al., 2004
; de Frias et al., 2007
; Raz et al., 2007
). Reliable between-person variation in cognitive change appears relatively late in life (e.g., around age 70), widening the gap between high-functioning and impaired older individuals (e.g., de Frias et al., 2007
). We found that aging magnifies the functional consequences of common genetic polymorphisms on executive functioning and working memory. We conclude that genetic factors boost individual differences in the aging of human cognition, and contribute to the marked heterogeneity in late-life cognitive functioning.
The authors declare that there is no conflict of interest.
This research was supported by the Max Planck Society, including a grant from the innovation fund of the Max Planck Society (M.FE.A.BILD0005). It also was supported by a grant from the German Federal Ministry for Research to the Berlin NeuroImaging Center (01GO0501). L.B. was supported by the Swedish Research Council (521-2007-2892) and the Swedish Brain Power. C.C. was supported by a Swiss National Foundation fellowship (No PBGE1-112883). I.N. was supported by a predoctoral fellowship of the International Max Planck Research School, The Life Course: Evolutionary and Ontogenetic Dynamics (LIFE). The authors thank Kirsten Becker, Silke Becker, Anita Günther, Karola Rockmann, and all the other research assistants for their help.
Bertolino, A., Rubino, V., Sambataro, F., Blasi, G., Latorre, V., Fazio, L., Caforio, G., Petruzzella, V., Kolachana, B., Hariri, A., Meyer-Lindenberg, A., Nardini, M., Weinberger, D. R., and Scarabino, T. (2006). Prefrontal-hippocampal coupling during memory processing is modulated by COMT val158met genotype. Biol. Psychiatry 60, 1250–1258.
Egan, M. F., Kojima, M., Callicott, J. H., Goldberg, T. E., Kolachana, B. S., Bertolino, A., Zaitsev, E., Gold, B., Goldman, D., Dean, M., Lu, B., and Weinberger, D. R. (2003). The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell 112, 257–269.
Ho, B. C., Milev, P., O’Leary, D. S., Librant, A., Andreasen, N. C., and Wassink, T. H. (2006). Cognitive and magnetic resonance imaging brain morphometric correlates of brain-derived neurotrophic factor Val66Met gene polymorphism in patients with schizophrenia and healthy volunteers. Arch. Gen. Psychiatry 63, 731–740.
Mattay, V. S., Goldberg, T. E., Fera, F., Hariri, A. R., Tessitore, A., Egan, M. F., Kolachana, B., Callicott, J. H., and Weinberger, D. R. (2003). Catechol O-methyltransferase val158-met genotype and individual variation in the brain response to amphetamine. Proc. Natl. Acad. Sci. U. S. A. 100, 6186–6191.
Meyer-Lindenberg, A., Straub, R. E., Lipska, B. K., Verchinski, B. A., Goldberg, T., Callicott, J. H., Egan, M. F., Huffaker, S. S., Mattay, V. S., Kolachana, B., Kleinman, J. E., and Weinberger, D. R. (2007). Genetic evidence implicating DARPP-32 in human frontostriatal structure, function, and cognition. J. Clin. Invest. 117, 672–682.
Tan, H. Y., Chen, Q., Sust, S., Buckholtz, J. W., Meyers, J. D., Egan, M. F., Mattay, V. S., Meyer-Lindenberg, A., Weinberger, D. R., and Callicott, J. H. (2007). Epistasis between catechol-O-methyltransferase and type II metabotropic glutamate receptor 3 genes on working memory brain function. Proc. Natl. Acad. Sci. U. S. A. 104, 12536–12541.
Xu, X., Mill, J., Zhou, K., Brookes, K., Chen, C. K., and Asherson, P. (2007). Family-based association study between brain-derived neurotrophic factor gene polymorphisms and attention deficit hyperactivity disorder in UK and Taiwanese samples. Am. J. Med. Genet. B Neuropsychiatr. Genet. 144, 83–86.