Mobile Software as a Medical Device (SaMD) for the Treatment of Epilepsy: Development of Digital Therapeutics Comprising Behavioral and Music-Based Interventions for Neurological Disorders
- 1Department of Neurology, University of Utah, Salt Lake City, UT, United States
- 2Department of Neurology, Weill Cornell Medicine, Cornell University, New York, NY, United States
- 3Department of Pediatrics, University of Utah, Salt Lake City, UT, United States
- 4Department of Medicinal Chemistry, College of Pharmacy, University of Utah, Salt Lake City, UT, United States
- 5Software Development Center, University of Utah, Salt Lake City, UT, United States
- 6Wild Out West, San Rafael, CA, United States
Digital health technologies for people with epilepsy (PWE) include internet-based resources and mobile apps for seizure management. Since non-pharmacological interventions, such as listening to specific Mozart's compositions, cognitive therapy, psychosocial and educational interventions were shown to reduce epileptic seizures, these modalities can be integrated into mobile software and delivered by mobile medical apps as digital therapeutics. Herein, we describe: (1) a survey study among PWE about preferences to use mobile software for seizure control, (2) a rationale for developing digital therapies for epilepsy, (3) creation of proof-of-concept mobile software intended for use as an adjunct digital therapeutic to reduce seizures, and (4) broader applications of digital therapeutics for the treatment of epilepsy and other chronic disorders. A questionnaire was used to survey PWE with respect to preferred features in a mobile app for seizure control. Results from the survey suggested that over 90% of responders would be interested in using a mobile app to manage their seizures, while 75% were interested in listening to specific music that can reduce seizures. To define digital therapeutic for the treatment of epilepsy, we designed and created a proof-of-concept mobile software providing digital content intended to reduce seizures. The rationale for all components of such digital therapeutic is described. The resulting web-based app delivered a combination of epilepsy self-care, behavioral interventions, medication reminders and the antiseizure music, such as the Mozart's sonata K.448. To improve long-term patient engagement, integration of mobile medical app with music and multimedia streaming via smartphones, tablets and computers is also discussed. This work aims toward development and regulatory clearance of software as medical device (SaMD) for seizure control, yielding the adjunct digital therapeutic for epilepsy, and subsequently a drug-device combination product together with specific antiseizure medications. Mobile medical apps, music, therapeutic video games and their combinations with prescription medications present new opportunities to integrate pharmacological and non-pharmacological interventions for PWE, as well as those living with other chronic disorders, including depression and pain.
Introduction
Among people with epilepsy (PWE) and their healthcare providers, there are overlapping needs for better control of: (1) seizures, (2) medication adherence and (3) comorbidities. According to the World Health Organization, there are 50–60 million people living with epilepsy world-wide, and only 70% of them respond to current treatments to control their seizures. In the United States alone, there are an estimated 3.4 million PWE, and approximately 150,000 people are diagnosed with epilepsy each year (Epilepsy Foundation, www.epilepsy.com). Newly-diagnosed patients have approximately 50% chance to become seizure free after taking their first antiseizure medication (Brodie et al., 2012, 2013; Chen et al., 2017). Recent work suggests that even after becoming seizure-free, over 60% of patients who discontinue taking antiseizure drugs experienced at least one relapse over a period of 3 years (Park et al., 2017). The diverse etiologies and complex mechanisms of epileptic seizures pose a challenge to reach long-term seizure freedom in approximately 20–30% of PWE (Golyala and Kwan, 2017; Tang et al., 2017). For patients with refractory epilepsy, possible non-pharmacological options to control seizures include dietary interventions (DeGiorgio et al., 2014; Kim et al., 2016; Martin et al., 2016), implantable neuromodulation devices, or brain surgery. Approximately 30% of PWE do not adhere to medication schedules, leading to decreased seizure control (Ettinger et al., 2009; Malek et al., 2017; O' Rourke and O' Brien, 2017). In addition to seizures, PWE often experience depression and anxiety as comorbidities, requiring additional interventions (Kanner, 2016). Taken together, there are multiple needs to develop new therapies with improved efficacy and clinical outcomes for PWE.
Digital health (also mobile health, mHealth, or eHealth,) is a branch of healthcare that employs internet, digital, and mobile technologies for improving health and/or treating specific medical conditions. Many digital health technologies are focused on wellness and health coaching, or disease self-management. mHealth comprises mobile medical apps which receive clearance (as SaMD) from the US Food and Drug Administration (FDA). Examples of SaMD are mobile medical apps, such as BlueStar® (developed by WellDoc) to improve control of glucose blood levels in patients with type 2 diabetes, or reSET® for substance use (addiction), developed by Pear Therapeutics. Music-based videogame MusicGlove and Jinxtronix neurorehabilitation system are examples of the FDA-cleared stroke therapies. Benefits of digital therapeutics include their ability to integrate patient behavior and lifestyle changes with pharmacotherapy (Bulaj, 2014; Bulaj et al., 2016; Mantani et al., 2017; McKennon et al., 2017).
Digital health technologies for PWE comprise mobile apps and internet-based resources delivering epilepsy self-management content (Pandher and Bhullar, 2014; Escoffery et al., 2018; Le Marne et al., 2018; Page et al., 2018). Survey-based studies suggest that PWE and their caregivers are interested in mHealth, but only a small fraction use mobile apps for seizure management (Liu et al., 2015, 2016; Leenen et al., 2016). Pandher and Bhullar reviewed features of 28 mobile apps for seizure management (Pandher and Bhullar, 2014). Content of mobile apps could be categorized into patient education (e.g., general information, seizure triggers, medications, first aid) and self-monitoring (seizure and medication tracking). The authors found relatively low quality of educational components in the apps, whereas seizure diaries were the most commonly incorporated features (Pandher and Bhullar, 2014). Recently, 20 mobile apps for epilepsy self-management were reviewed with respect to self-management functions and behavior change strategies, as well as evaluated for their engagement, functionality, esthetics, information quality and satisfaction using the Mobile App Rating Scale (MARS) (Escoffery et al., 2018). An example of online self-management platform for epilepsy patients is WebEase (Epilepsy Awareness, Support and Education) delivering three modules focused on medications, stress and sleep (DiIorio et al., 2009a,b). Another web-based intervention is the Epilepsy Journey, targeting executive function deficits in adolescents with epilepsy (Modi et al., 2017). Feasibility of mobile and internet-based delivery of psychosocial interventions and cognitive behavioral therapy for PWE further emphasizes opportunities for digital interventions (Hixson et al., 2015; Gandy et al., 2016; Glynn et al., 2016). Wearables intended to detect and predict seizures have been developed, for example SmartWatch and the FDA-cleared Embrace by Empatica. To the best of our knowledge there are no published studies on effects of digital health technologies on reduction of seizure frequency.
For PWE, digital health technologies offer opportunities to improve therapy outcomes by integrating prescription medications with specific musical compositions (Bulaj, 2014). As summarized in Table 1 and described in the Results section, there are several published reports on antiseizure effects of Mozart's sonata K.448 on reducing seizure frequencies and epileptiform discharges in PWE. Antiseizure effects of K.448 were observed in children with refractory epilepsy (Lin et al., 2011a), and in those after first unprovoked seizures (Lin et al., 2014a). The clinical findings are also supported by animal studies suggesting that Mozart's K.448 can reduce seizure frequencies in rats (Lin et al., 2013), upregulates expression of brain-derived neurotrophic factor (BDNF) in rats (Xing et al., 2016a,b,c), reduces cognitive impairment in status epilepticus rats (Xing et al., 2016b), increases brain levels of dopamine in rats (Tasset et al., 2012), and modulates expression of several genes involved in neurotransmission in the hippocampus and the forebrain cortex in mice (Meng et al., 2009). The possible mechanism by which music exerts antiseizure effects may include neuromodulation of the parasympathetic system (Lin et al., 2013; Dastgheib et al., 2014).
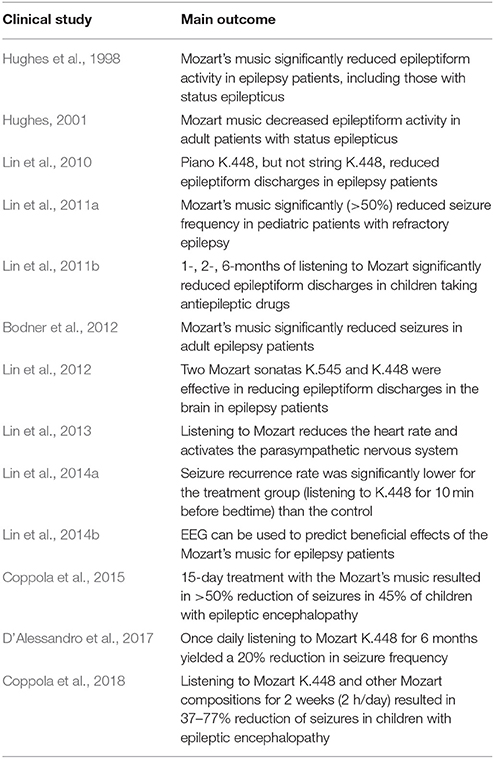
Table 1. A summary of clinical studies of listening to specific Mozart's compositions on epileptic seizures and epileptiform discharges.
In this work, we describe initial steps in development of digital therapeutics for epilepsy, including the survey-based study and design of prototype mobile software intended to reduce seizures. We provide a rationale for mobile medical app content which integrates antiseizure music, epilepsy self-care and elements of cognitive behavioral therapy. As illustrated in Figure 1, our long term goal is to develop SaMD as adjunct digital therapeutic for the treatment of epilepsy, followed by integration of specific antiseizure drugs with SaMD, yielding, from a regulatory perspective, a drug-device combination product. The drug-SaMD combination product offers means to: (1) integrate pharmacological and behavioral therapies with self-care, (2) create new personalized treatments for epilepsy, and (3) improve medication adherence and patient engagement. Our work has implications beyond epilepsy, since combining prescription medications and music-based interventions is also applicable to the treatment of depression (Schriewer and Bulaj, 2016), pain (Chai et al., 2017) and potentially other neurological disorders (Sihvonen et al., 2017).
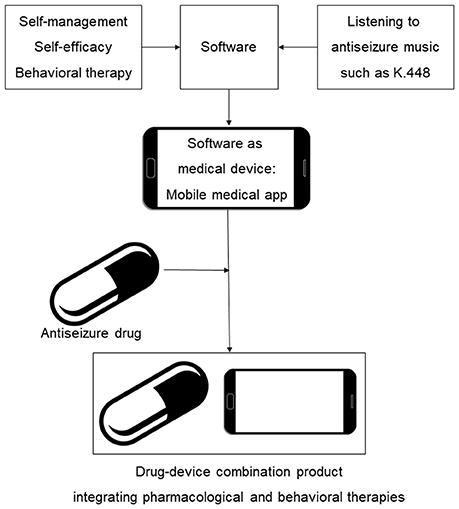
Figure 1. Scheme illustrating integration of behavioral interventions, self-care (self-management and self-efficacy) and antiseizure music into mobile medical app (SaMD) and a drug-device combination product for epilepsy. An incentive for clinical development and regulatory approval of such digital therapeutics and their combinations with brand and generic drugs is the potential for long-lasting intellectual property protection based on copyrights of creative works and software (Bulaj, 2014; Bulaj et al., 2016).
Methods
The Survey Study
The study was conducted in the University of Utah Adult Comprehensive Epilepsy Clinic. The institutional review board (IRB) at University of Utah reviewed and approved the study. The study was determined to be “exempt” by the IRB due to its minimal risk to participants. The IRB approved an authorization cover letter for participants to sign prior to completing the study questionnaire. The study information was kept in secured manner and electronic records of patients were password protected. The questionnaire was designed and consisted of seven questions related to preferences in using mobile apps for seizure management and control (Supplementary Information). The questionnaire collection forms were created for each participant and information was directly captured in the electronic clinical data management tool REDCap (Research Electronic Data Capture Software). This study was conducted between months June and August 2017, and involved PWE aged 18 years and older who were regular smartphone users. Patients without access to smartphones, and/or who were cognitively impaired were excluded from the study population. A total of 40 individuals participated in this study.
After signing consent, each participant was first provided with brief introduction about the purpose of this study. Any person who did not agree to participate in this study was excluded. Participant's protected health information (PHI) was linked to their questionnaire to avoid data duplication for which the IRB has approved an authorization cover letter for participants to sign prior to enrollment. Questions were explained to the participants by the moderator, who was a student trained in qualitative research methods. The epilepsy attending and/or study coordinator and/or a student were present during data collection. The participants were asked to select the options that they felt were most beneficial and desirable for them, and features they wanted in the mobile app. The questionnaire answers were completed by the participants using the REDCap. While the authors had a positive attitude toward mobile app, they strived to remain neutral in conversation with the participants. Data analysis and reports were viewed on REDCap Stats and Charts.
Design of Mobile Software Prototype
Using PubMed, literature search was performed to identify clinical studies of non-pharmacological interventions that reported seizure reduction in PWE. The following key words were used alone and in combination with “epilepsy” or “epileptic seizures”: self-management, self-efficacy, self-care, psychosocial, cognitive behavioral therapy, educational intervention, web-based intervention, internet, music, Mozart. Information from the published studies reporting a reduction of seizure frequency was analyzed and incorporated into software content. When designing the prototype mobile software we hypothesized that the duration of the digital therapy would last preferably 1 year, or longer, during which time a patient would be engaged with the mobile app for approximately 10 min daily. Therefore, user experience (UX) interface, interactivity, gamification and novel daily content were important factors to maximize patient's engagement. To create a prototype of the mobile software, a web-based version was built using HTML5 and hosted on a shared-server Linux platform. Visual displays of the digital content were discussed among software and UX engineers and clinical team members.
Results
The Survey Study
The main purpose of our survey study was to evaluate the patient's interest in using a mobile app for seizure control and self-care. Our questionnaire was focused on questions about preferred features in a mobile app related to seizure management, wellbeing, self-awareness, empowerment, and engagement in the therapy. All questions and answers are provided in the Supplementary Information. A total of 40 individuals participated in this study. The survey results show that over 90% of patients were interested in using a mobile app to help manage their seizures. In relation to monitoring epilepsy, 85% of patients were interested in diary to record date of their seizures, 73% were interested in recording type of their seizures, 78% were interested in logging the missed dosages of their medications. Regarding automated reminders, a majority was interested in reminders to keep their follow up appointments (80%), to refill their medications (73%), to take their medication on time (68%).
In relation to understanding their disease, 68% of responders were interested in delivery of brief epilepsy and seizure-related information (Figure 2), 84% were interested about how their feelings and environment affect their epilepsy, 63% were interested in learning about feelings related to their epilepsy, while 42% were interested in being inspired by quotations and imagery. Additionally, 5% of participants did not find informative features applicable to them and therefore left this section blank.
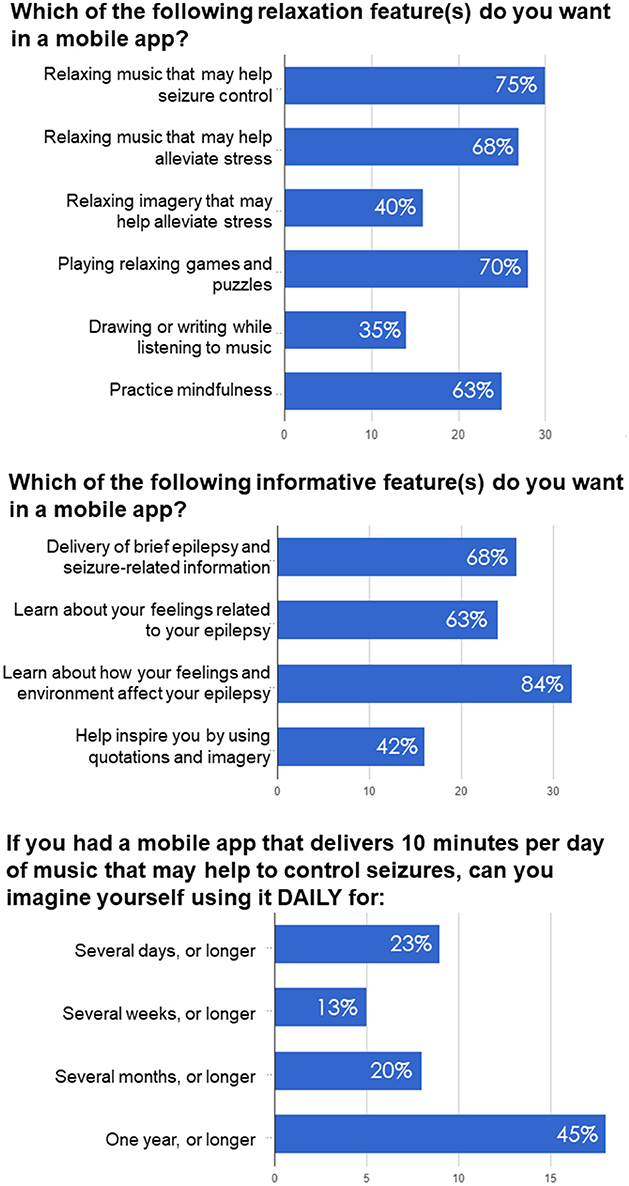
Figure 2. Examples of questions and responses in the survey-based study examining preferences of PWE regarding antiseizure music-based intervention, self-awareness and self-care delivered via mobile app.
Two questions related to music-based intervention were focused on seizure control and comparing music with other relaxation techniques like imagery, games and puzzles, and practicing mindfulness. About 75% of responders were interested in music that may help seizure control and 68% were interested in relaxing music that may help alleviate stress, while 40% of patients were interested in relaxing imagery that may help alleviate stress (Figure 2). 70% of patients were interested in playing relaxing games and puzzles, while 35% were interested in drawing or writing while listening to music. About 63% were interested in practicing mindfulness.
To determine potential adherence to music-based intervention, we asked participants how long they would be willing to listen to antiseizure music, if it was delivered daily for 10 min. Previous studies on the effects of Mozart music on seizure reduction suggested that music intervention was effective when delivered for at least 1 month, while most studies tested antiseizure effects of music delivered for either 6 or 12 months (Lin et al., 2011a; Bodner et al., 2012; Dastgheib et al., 2014; D'Alessandro et al., 2017). As shown in Figure 2, a majority of responders (65%) imagined using it for several months or longer (including 45% who imagined such therapy lasting for 1 year or longer). 13% of responders could use it for several weeks or longer, while 23% for several days or longer. In summary, the questionnaire responses confirmed PWE's interest in using a mobile app for seizure control, management and self-care.
A Rationale for Integrating Non-pharmacological Interventions Into Mobile Software
PubMed literature search identified non-pharmacological interventions that resulted in reducing the frequency of seizures in PWE (Tables 1, 2). Our hypothesis is that incorporating non-pharmacological interventions and self-care into mobile software can yield digital therapeutics (SaMD) for epilepsy (Figure 1). Clinical studies showed that daily listening to Mozart's sonata K.448 significantly reduced frequency of epileptic seizures and/or epileptiform discharges in adult and pediatric patients with epilepsy (Table 1). Listening to K.448 was effective in reducing seizure frequency even after 1 month, and further improved seizure frequency over 6 month treatment time (Lin et al., 2011a). Studies also showed that additional Mozart's compositions, including K.207, K.218, K.314, K.482, K. 545, and K.551 had positive effects on children with epilepsy, including reduction of epileptiform discharges and reduction of seizure frequency (Lin et al., 2012; Coppola et al., 2015, 2018). Possible mechanisms by which music may exert its antiseizure effect include activation of the parasympathetic system (Lin et al., 2013), stabilization of the hypothalamic-pituitary-adrenal (HPA) axis (Maguire and Salpekar, 2013; Wulsin et al., 2016), or include dopaminergic signaling through D2-like receptors (Salimpoor et al., 2011; Bozzi and Borrelli, 2013).
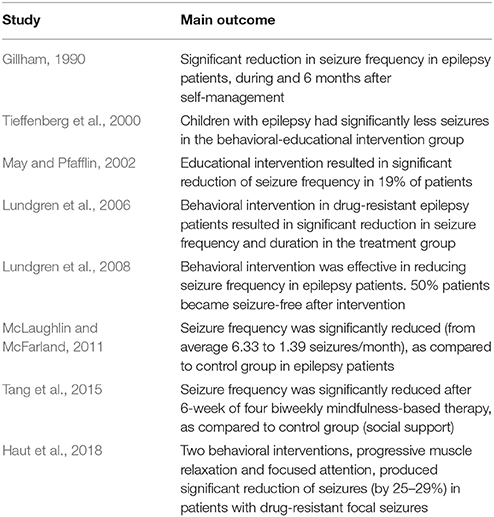
Table 2. A summary of clinical studies showing reduction of seizures following behavioral interventions.
Studies of psychosocial interventions, behavioral cognitive therapy and self-management for PWE (Kotwas et al., 2017; Michaelis et al., 2017) suggest that self-care practices may lead to reduction of seizure frequency (Mittan, 2009; Edward et al., 2015; Table 2, Figure 3). In the review of psychosocial interventions in epilepsy (Mittan, 2009), the author found five studies reporting positive findings out of seven studies that measured seizure control. A significant reduction of seizures in older adult patients with epilepsy was observed at 3-month follow up after 6-week cognitive behavioral therapy intervention (McLaughlin and McFarland, 2011). Positive results in seizure reduction were observed in randomized trial of behavioral interventions in patients with the refractory epilepsy (Gillham, 1990). Another randomized trial of behavioral therapy (acceptance and commitment therapy) showed a significant reduction of seizure frequency in patients with the refractory epilepsy (Lundgren et al., 2008). Behavioral and educational interventions were effective in reducing seizures in both pediatric and adult populations (Spector et al., 1999, 2001; Tieffenberg et al., 2000; May and Pfafflin, 2002), also emphasizing importance of self-efficacy, knowledge, understanding and management of emotional health (Spector et al., 2000, 2001; McLaughlin and McFarland, 2011). Noteworthy, targeting the HPA axis for reduction of seizures has been proposed (Maguire and Salpekar, 2013; Wulsin et al., 2016). As summarized in Figure 3, when designing the prototype mobile software for epilepsy patients, we incorporated key behavioral and self-care aspects that support seizure control through a combination of education, awareness and behavioral interventions.
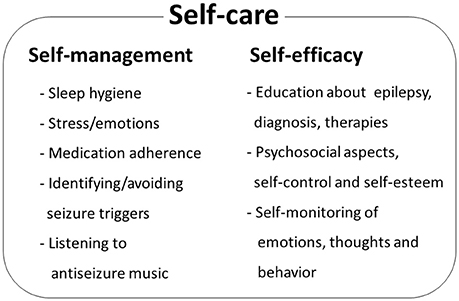
Figure 3. Epilepsy self-care comprises self-management components and patient's self-efficacy. Patient's behaviors and life-style may improve seizure control by identifying and managing seizure triggers. Patient's perceived self-control of epileptic seizures, awareness of behavior and emotional status and self-esteem contribute to health-related locus of control and self-efficacy. Psychosocial and behavioral therapy studies identify key elements that contribute to seizure control. Additional patient behavioral component is daily listening to antiseizure music, such as Mozart's sonata K.448.
Design of the Prototype Mobile Software
We designed and created the proof-of-concept software, which consisted of two main modules: (1) self-care (education and self-examining), and (2) leisure activities while listening to a total of 10 min of antiseizure music. As shown in Figure 4, key elements of the proof-of-concept mobile software included: (1) welcoming front page, (2) epilepsy-focused educational content, (3) self-management provided as self-examination, (4) improving self-esteem and self-efficacy, (5) listening to antiseizure music, (6) gamification, and (7) summary page. Examples of web-based screens are illustrated in Figure 5. Opening page was designed to welcome a patient while summarizing cumulative “score” of being engaged with the mobile app (Figure 4). Entering next pages automatically started playing antiseizure music, and for the proof-of-concept mobile software we looped the first movement of Mozart's sonata K.448. The next page provided “daily educational information” related to epilepsy and focused on the epilepsy knowledge, therapy, self-care and advances in clinical research. Next several pages were designed as self-examination of self-care components related to seizure control and quality of life (Figure 4). Interactive self-examining was delivered by structuring questions as introspective: “Have I experienced….” or “How was my sleep last night?” encouraging patient self-reflection before answering these questions. Self-examination was focused on enjoyable activities, relaxation, daily gratitude (reinforcing positive attitude and self-esteem), potential seizure triggers (stress, lack of sleep, emotions, others), and medication compliance reminders. After self-examination, the remaining time (of a total of 10 min) of listening to the antiseizure music could be spent on leisurely or creative activities chosen by the patient.
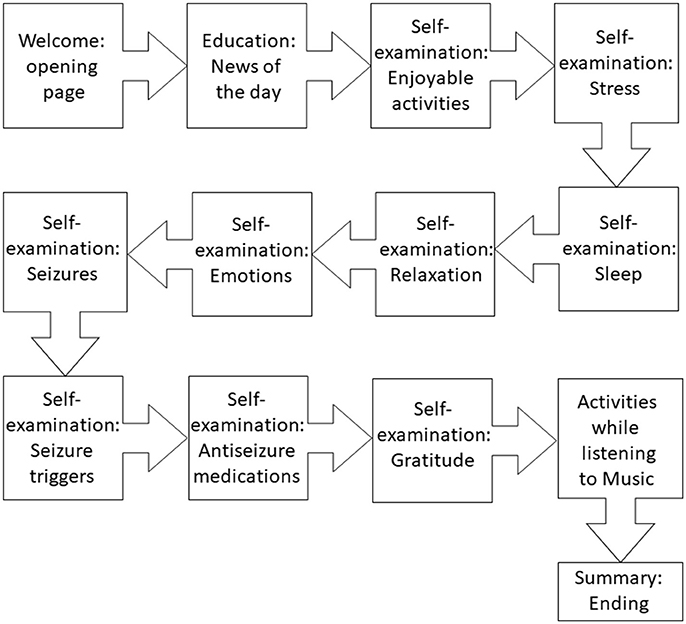
Figure 4. Flow of the proof-of-concept mobile software for epilepsy patients. The interaction between a patient and the software was intended to last 10 min daily.
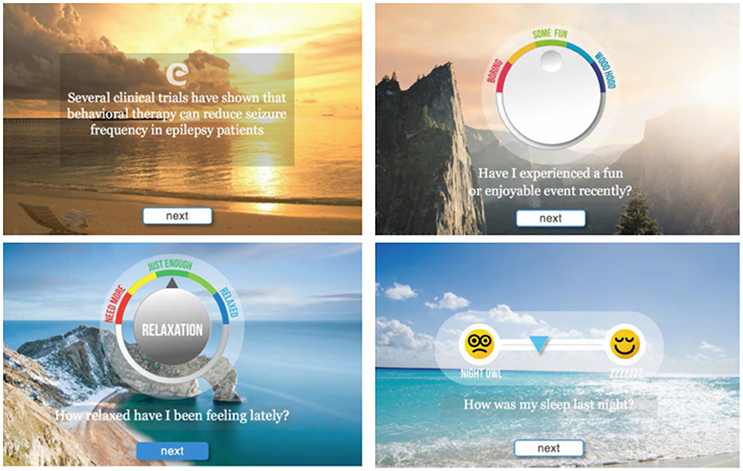
Figure 5. Representative screen shots from the proof-of-concept mobile software for epilepsy patients. Background images are presented as sliders and provide additional 3D perception.
The prototype mobile application was designed to create an engaging and intuitive user experience. The web-based prototype was built using HTML5 and hosted on a shared-server Linux platform. The interactions were primarily audio and visual, including progress feedback that can be monitored/recorded using emoticon-type symbols as opposed to a more traditional journaling process. As illustrated in Figure 5, the application's user interface was focused on making the experience easy and friendly, with a clean and simple modern interface.
Discussion
Despite increasing number of digital health technologies for seizure management, we are not aware of development of mobile medical apps intended to reduce frequency of seizures in epilepsy patients. Our survey suggest that about two-thirds of PWE could see themselves using such mobile software for at least several months. Patients preferred automated features for different aspects of seizure and disease management, and were also interested in the relaxation features of the app. One interesting finding was that our subjects' interest was more in passively listening to music than actively using imagery. They were also more interested in playing games or puzzles, as compared to writing or drawing while listening to music. We concluded that in our subject population there was less interest in relaxation techniques that used mental creativity (drawing, writing or using imagery). There was more interest in relaxation techniques consisting of observation (practicing mindfulness) or immersing passively by listening to music, or engaging reactively in puzzles and games, as compared to those techniques involving mental creativity. The reasons for preferences toward more passive activities are not clear at this time, but it may be that such relaxation techniques might increase brain stem parasympathetic activity, which in turn may have some anti-seizure effects. Bodner and colleagues reported that antiseizure music was also effective in reducing seizures when delivered during sleeping (Bodner et al., 2012), suggesting that for PWE who favor less engagement there are opportunities to develop more passive strategies (like streaming antiseizure music during sleep).
Music is a non-pharmacological modality that produces pleiotropic physiological effects, including activation of the dopaminergic system and D2 receptors (Salimpoor et al., 2011). Music-evoked neurochemical changes in the brain suggest therapeutic potential in affective and neurological disorders (Koelsch, 2010, 2014; Chanda and Levitin, 2013). Increasing evidence from preclinical and clinical studies supports the development of digital therapeutics for epilepsy delivering specific musical compositions. However, listening daily to the same music (e.g., Mozart's K.448) for several months may produce undesirable and even maladaptive effects including a lack of interest, irritation and “desensitization” of physiological effects. Results from a recent animal study suggest that the CNS effects of K.448 may be in part due to the so called Mozart rhythm effect (Xing et al., 2016c). To identify musical compositions similar to Mozart's sonata K.448, a neurologist/clinical neurophysiologist JR Hughes used computer analysis of periodicity and melodic lines in 330 compositions of Mozart, 155 of JS Bach, 61 of Beethoven, 58 of Chopin and 23 of Wagner, and selected 25 compositions with the highest values of long-lasting periodicities (Hughes, 2001, 2002), expanding a potential repertoire of music for clinical testing for antiseizure properties.
Based on our observations and other studies (van Andel et al., 2011; Walker et al., 2012, 2014), it is apparent that in addition to PWE, their caregivers and close friends may also benefit from using mobile software designed for epilepsy self-care. Social support needs of PWE and caregivers offer future opportunities to expand the mobile app content in which: (1) PWE and their closest support group can share access to learning about epilepsy, self-management and wellbeing, (2) PWE could connect with their primary caregivers, while still maintaining the autonomy to control their individual use of the app, (3) caregivers could update seizures recordings for those that the patient might not have been aware of, (4) PWE and their loved ones can socially interact hence minimizing the feeling of isolation that is known to often accompany those living with epilepsy.
Mobile software for epilepsy patients is a non-pharmacological modality that can be easily combined with other therapies (Figure 6). The most apparent combinations are with antiseizure medications (Bulaj, 2014), or with dietary interventions such as ketogenic and low-glycemic diets (DeGiorgio et al., 2014; Kim et al., 2016; Martin et al., 2016). A rationale for combining mobile software with pleiotropic natural products including n-3 polyunsaturated fatty acids was previously discussed (DeGiorgio et al., 2014; Bulaj et al., 2016). For patients with refractory epilepsy, mobile medical app delivering self-care and antiseizure music can be validated for use with neuromodulation devices like vagus nerve and deep brain stimulation, VNS and DBS, respectively, although additional safety and efficacy studies and regulatory authorization would be required before marketing such combination therapies. Recently, we described music streaming as an adjunct digital therapy for depression, anxiety and bipolar spectrum disorders (Schriewer and Bulaj, 2016). Music and multimedia streaming of the antiseizure digital content can complement the use of a mobile medical app for seizure control. In addition to increasing patient engagement, web-based streaming offers an additional safety feature by mitigating potentially stressful events such as losing or breaking a mobile phone, tablet, or laptop that hosts the SaMD. Technological advancements in wearables that provide real-time physiological feedback (e.g., smart watches by Empatica, or mobile EEG systems by Emotiv, Muse, or NeuroSky) may be used to optimize streamed content, similarly to the strategy used for patients with depression (Ramirez et al., 2015). The development of clinically-validated, multimedia streaming with subsequent integration with the mobile app for PWE are longer-term prospects.
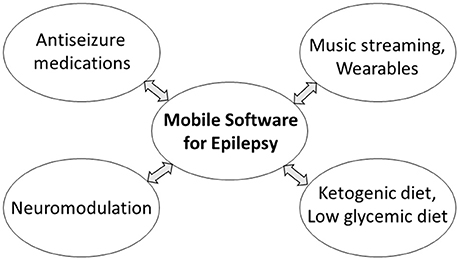
Figure 6. Possible combinations of mobile medical app for the treatment of epilepsy with pharmacological and non-pharmacological interventions. Some combinations may require additional safety and efficacy studies and regulatory authorization before marketing.
In the present study, we describe the initial stage of developing prescription digital therapeutic for the treatment of epilepsy in patients already taking antiseizure medications. Due to multiple challenges in developing such innovative medical technology, we exercise step-wise and iterative approach which started with the survey and the proof-of-concept prototype design (this work). Before any clinical testing, the web-based prototype will be converted into an alpha version of the mobile medical app, due to current regulatory guidelines. The step-wise development plan includes: (1) pilot feasibility study of the alpha version, (2) optimization based on feedback from the pilot study, yielding beta version, (3) a randomized, controlled pivotal (efficacy) trial of the beta version. Since the mobile medical app is intended to be used as add-on digital therapeutic, two main clinical outcomes to be studied will be seizure frequency and medication adherence. For the alpha version of the mobile medical app, the software will be built using best-of-breed software engineering standards and the tools necessary to support highly secure and readily available mobile software. While the primary experience will be native to the mobile device, the software will include a server-side component used to manage software updates and limited user data. The system will comply with all relevant FDA regulations and be HIPAA compliant. Both Android and iOS devices will be included in the initial release. The regulatory pathway will follow the recommendations in the FDA guidance document that can be found at www.fda.gov/MedicalDevices/DigitalHealth/MobileMedicalApplications/default.htm. Anticipated challenges during development include long-term patient engagement, privacy protection and cybersecurity.
This paper also describes the development strategy for merging pharmacological and behavioral therapies for PWE by means of drug-device combination products in which an antiseizure drug works together with a mobile medical app (Figures 1, 6). Companies including Pear Therapeutics and Akili Interactive already develop clinically validated mobile apps and videogames delivering disease-specific behavioral therapy as stand-alone “prescription digital therapeutics,” or to be used with prescription medications. This strategy illustrates broader impact of digital therapeutics allowing integration of self-care and behavioral therapies with pharmaceutical drugs (Bulaj et al., 2016). From a US FDA regulatory perspective, approval of a drug-device combination product incorporating a mobile medical app for use with a drug typically involves first completing a pilot investigation to confirm the feasibility of the approach. After device refinements, a pivotal clinical investigation is performed to reasonably establish the safety and effectiveness of the drug-device combination product. An incentive for innovating therapies using digital therapeutics is their copyright-based intellectual property protection, applicable even to combinations with generic drugs (Bulaj, 2014). Regulatory processes for medical devices vary from country to country, and as digital therapy is a relatively new field, regulatory requirements for developing and obtaining regulatory authorization to market mobile software as a medical device continue to evolve. Challenges associated with developing digital therapeutics include: (1) mitigating the gap between rapidly changing technologies and the slower-pace of clinical development, (2) mitigating patient risks including stress associated with losing/breaking a digital device, (3) incorporating robust cybersecurity measures, (4) ensuring patient engagement, and (5) health care system implementation and reimbursement.
Digital therapeutics delivering multimodal interventions, including drug-device combination therapies, may benefit patients with neurological and other chronic medical conditions (Bulaj, 2014; Bulaj et al., 2016) including treatment of depression, pain, arthritis, and diabetes, given similar comorbidities. Music and mobile apps, such as SuperBetter (Roepke et al., 2015), MoodHacker (Birney et al., 2016), as well as web-based interventions (Merry et al., 2012; Buntrock et al., 2016) have been shown to reduce or prevent depressive symptoms (Firth et al., 2017; Leubner and Hinterberger, 2017), even in patients with refractory depression (Mantani et al., 2017). Virtual reality technologies improve pain relief and decrease opioid use (McSherry et al., 2017; Tashjian et al., 2017). Music-based interventions to manage acute and chronic pain may also show promise (Chai et al., 2017). Listening to the Mozart K.448 also produced beneficial clinical effects for people with schizophrenia taking antipsychotic drugs (He et al., 2018). These and other studies suggest that converting non-pharmacological interventions into mobile software as medical device and subsequent integration with antiseizure, antidepressant and analgesic medications is an attractive strategy for improving therapy outcomes in several neurologic disorders (Figure 7).
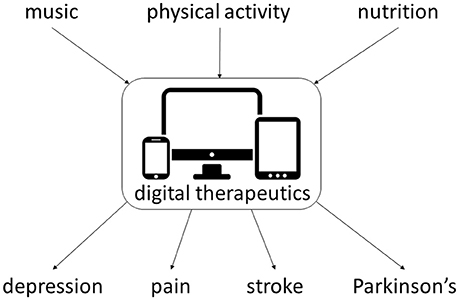
Figure 7. Examples of non-pharmacological interventions amenable for integration with digital therapeutics for chronic medical conditions. Multiple clinical studies support integration of such multimodal interventions for depression (Pirbaglou et al., 2016; Leubner and Hinterberger, 2017; Toups et al., 2017), pain (Cooney et al., 2013; Chai et al., 2017; Qaseem et al., 2017; Toups et al., 2017), stroke (Pollock et al., 2014a,b; Sihvonen et al., 2017), and Parkinson's disease (Seidl et al., 2014; Bloem et al., 2015; Roeder et al., 2015).
Author Contributions
PA, CB, MS, and GB conceived the project and defined digital content of the prototype mobile software for people with epilepsy; PA, CB, MS, GB, JJ, and DS designed the prototype mobile software; PA, CB, MS, and GB designed the survey questionnaire; PA, LF, FA, MG, MH, and KN collected survey results; PA, LF, and FA analyzed the survey results; PA, CB, MS, LF, FA, MG, MH, and KN reviewed the literature and discussed the results; PA, CB, MS, LF, FA, MG, MH, KN, JJ, and DS wrote and edited the manuscript.
Conflict of Interest Statement
CB and GB are co-founders, officers and board members in Epicadence, Public Benefit Corporation, focused on development of mobile software for epilepsy patients. JJ is an officer in Epicadence, PBC, and a cofounder and an officer in Stretto Consulting. PA and MS are consultants to Epicadence, Public Benefit Corporation. PA, CB, MS, and GB are co-inventors on patent-pending “Multimodal Platform for Treating Epilepsy” licensed to Epicadence PBC. The patent claims describe methods and uses of coupling music and multimedia streaming with mobile app to reduce seizures in people with epilepsy. DS is a cofounder and an officer in WildOutWest.
The other authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Spencer Walker from the Software Development Center for his assistance with creating proof-of-concept mobile software. We thank Phil Triolo for helpful discussions on regulatory requirements for mobile devices, and for critical reading of the manuscript. We thank Margo Thurman for her comments on the manuscript. This work was supported by internal funding from the University of Utah.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnhum.2018.00171/full#supplementary-material
Abbreviations
HPA, the hypothalamic-pituitary-adrenal; K.448, Mozart's sonata K.448; PWE, people with epilepsy; SaMD, software as a medical device; UX, user experience.
References
Birney, A. J., Gunn, R., Russell, J. K., and Ary, D. V. (2016). MoodHacker mobile web app with email for adults to self-manage mild-to-moderate depression: randomized controlled trial. JMIR Mhealth Uhealth 4, e8. doi: 10.2196/mhealth.4231
Bloem, B. R., de Vries, N. M., and Ebersbach, G. (2015). Nonpharmacological treatments for patients with Parkinson's disease. Mov. Disord. 30, 1504–1520. doi: 10.1002/mds.26363
Bodner, M., Turner, R. P., Schwacke, J., Bowers, C., and Norment, C. (2012). Reduction of seizure occurrence from exposure to auditory stimulation in individuals with neurological handicaps: a randomized controlled trial. PLoS ONE 7:e45303. doi: 10.1371/journal.pone.0045303
Bozzi, Y., and Borrelli, E. (2013). The role of dopamine signaling in epileptogenesis. Front. Cell. Neurosci. 7:157. doi: 10.3389/fncel.2013.00157
Brodie, M. J., Barry, S. J., Bamagous, G. A., and Kwan, P. (2013). Effect of dosage failed of first antiepileptic drug on subsequent outcome. Epilepsia 54, 194–198. doi: 10.1111/j.1528-1167.2012.03722.x
Brodie, M. J., Barry, S. J., Bamagous, G. A., Norrie, J. D., and Kwan, P. (2012). Patterns of treatment response in newly diagnosed epilepsy. Neurology 78, 1548–1554. doi: 10.1212/WNL.0b013e3182563b19
Bulaj, G. (2014). Combining non-pharmacological treatments with pharmacotherapies for neurological disorders: a unique interface of the brain, drug-device, and intellectual property. Front Neurol. 5:126. doi: 10.3389/fneur.2014.00126
Bulaj, G., Ahern, M. M., Kuhn, A., Judkins, Z. S., Bowen, R. C., and Chen, Y. (2016). Incorporating natural products, pharmaceutical drugs, self-care and digital/mobile health technologies into molecular-behavioral combination therapies for chronic diseases. Curr. Clin. Pharmacol. 11, 128–145. doi: 10.2174/1574884711666160603012237
Buntrock, C., Ebert, D. D., Lehr, D., Smit, F., Riper, H., Berking, M., et al. (2016). Effect of a web-based guided self-help intervention for prevention of major depression in adults with subthreshold depression: a randomized clinical trial. JAMA 315, 1854–1863. doi: 10.1001/jama.2016.4326
Chai, P. R., Carreiro, S., Ranney, M. L., Karanam, K., Ahtisaari, M., Edwards, R., et al. (2017). Music as an adjunct to opioid-based analgesia. J. Med. Toxicol. 13, 249–254. doi: 10.1007/s13181-017-0621-9
Chanda, M. L., and Levitin, D. J. (2013). The neurochemistry of music. Trends Cogn. Sci. 17, 179–193. doi: 10.1016/j.tics.2013.02.007
Chen, Z., Brodie, M. J., Liew, D., and Kwan, P. (2017). Treatment outcomes in patients with newly diagnosed epilepsy treated with established and new antiepileptic drugs: a 30-year longitudinal cohort study. JAMA Neurol. 75, 279–286. doi: 10.1001/jamaneurol.2017.3949
Cooney, G. M., Dwan, K., Greig, C. A., Lawlor, D. A., Rimer, J., Waugh, F. R., et al. (2013). Exercise for depression. Cochrane Database Syst. Rev. 2013:CD004366. doi: 10.1002/14651858.CD004366.pub6
Coppola, G., Operto, F. F., Caprio, F., Ferraioli, G., Pisano, S., Viggiano, A., et al. (2018). Mozart's music in children with drug-refractory epileptic encephalopathies: comparison of two protocols. Epilepsy Behav. 78, 100–103. doi: 10.1016/j.yebeh.2017.09.028
Coppola, G., Toro, A., Operto, F. F., Ferrarioli, G., Pisano, S., Viggiano, A., et al. (2015). Mozart's music in children with drug-refractory epileptic encephalopathies. Epilepsy Behav. 50, 18–22. doi: 10.1016/j.yebeh.2015.05.038
D'Alessandro, P., Giuglietti, M., Baglioni, A., Verdolini, N., Murgia, N., Piccirilli, M., et al. (2017). Effects of music on seizure frequency in institutionalized subjects with severe/profound intellectual disability and drug-resistant epilepsy. Psychiatr. Danub. 29(Suppl. 3), 399–404.
Dastgheib, S. S., Layegh, P., Sadeghi, R., Foroughipur, M., Shoeibi, A., and Gorji, A. (2014). The effects of Mozart's music on interictal activity in epileptic patients: systematic review and meta-analysis of the literature. Curr. Neurol. Neurosci. Rep. 14:420. doi: 10.1007/s11910-013-0420-x
DeGiorgio, C. M., Miller, P. R., Harper, R., Gornbein, J., Schrader, L., Soss, J., et al. (2014). Fish oil (n-3 fatty acids) in drug resistant epilepsy: a randomised placebo-controlled crossover study. J. Neurol. Neurosurg. Psychiatry 86, 65–70. doi: 10.1136/jnnp-2014-307749
DiIorio, C., Escoffery, C., McCarty, F., Yeager, K. A., Henry, T. R., Koganti, A., et al. (2009a). Evaluation of webease: an epilepsy self-management website. Health Educ. Res. 24, 185–197. doi: 10.1093/her/cyn012
DiIorio, C., Escoffery, C., Yeager, K. A., McCarty, F., Henry, T. R., Koganti, A., et al. (2009b). Webease: development of a web-based epilepsy self-management intervention. Prev. Chronic Dis. 6, A28. Available online at: www.cdc.gov/pcd/issues/2009/Jan/pdf/07_0263.pdf
Edward, K. L., Cook, M., and Giandinoto, J. A. (2015). An integrative review of the benefits of self-management interventions for adults with epilepsy. Epilepsy Behav. 45, 195–204. doi: 10.1016/j.yebeh.2015.01.026
Escoffery, C., McGee, R., Bidwell, J., Sims, C., Thropp, E. K., Frazier, C., et al. (2018). A review of mobile apps for epilepsy self-management. Epilepsy Behav. 81, 62–69. doi: 10.1016/j.yebeh.2017.12.010
Ettinger, A. B., Manjunath, R., Candrilli, S. D., and Davis, K. L. (2009). Prevalence and cost of nonadherence to antiepileptic drugs in elderly patients with epilepsy. Epilepsy Behav. 14, 324–329. doi: 10.1016/j.yebeh.2008.10.021
Firth, J., Torous, J., Nicholas, J., Carney, R., Pratap, A., Rosenbaum, S., et al. (2017). The efficacy of smartphone-based mental health interventions for depressive symptoms: a meta-analysis of randomized controlled trials. World Psychiatry 16, 287–298. doi: 10.1002/wps.20472
Gandy, M., Karin, E., Fogliati, V. J., McDonald, S., Titov, N., and Dear, B. F. (2016). A feasibility trial of an Internet-delivered and transdiagnostic cognitive behavioral therapy treatment program for anxiety, depression, and disability among adults with epilepsy. Epilepsia 57, 1887–1896. doi: 10.1111/epi.13569
Gillham, R. A. (1990). Refractory epilepsy: an evaluation of psychological methods in outpatient management. Epilepsia 31, 427–432. doi: 10.1111/j.1528-1157.1990.tb05498.x
Glynn, P., Eom, S., Zelko, F., and Koh, S. (2016). Feasibility of a mobile cognitive intervention in childhood absence epilepsy. Front. Hum. Neurosci. 10:575. doi: 10.3389/fnhum.2016.00575
Golyala, A., and Kwan, P. (2017). Drug development for refractory epilepsy: the past 25 years and beyond. Seizure 44, 147–156. doi: 10.1016/j.seizure.2016.11.022
Haut, S. R., Lipton, R. B., Cornes, S., Dwivedi, A. K., Wasson, R., Cotton, S., et al. (2018). Behavioral interventions as a treatment for epilepsy: a multicenter randomized controlled trial. Neurology 90, e963–e70. doi: 10.1212/WNL.0000000000005109
He, H., Yang, M., Duan, M., Chen, X., Lai, Y., Xia, Y., et al. (2018). Music intervention leads to increased insular connectivity and improved clinical symptoms in schizophrenia. Front. Neurosci. 11:744. doi: 10.3389/fnins.2017.00744
Hixson, J. D., Barnes, D., Parko, K., Durgin, T., Van Bebber, S., Graham, A., et al. (2015). Patients optimizing epilepsy management via an online community: the POEM Study. Neurology 85, 129–136. doi: 10.1212/WNL.0000000000001728
Hughes, J. R. (2002). The mozart effect: additional data. Epilepsy Behav. 3, 182–184. doi: 10.1006/ebeh.2002.0329
Hughes, J. R., Daaboul, Y., Fino, J. J., and Shaw, G. L. (1998). The “Mozart effect” on epileptiform activity. Clin. Electroencephalogr. 29, 109–119. doi: 10.1177/155005949802900301
Kanner, A. M. (2016). Management of psychiatric and neurological comorbidities in epilepsy. Nat. Rev. Neurol. 12, 106–116. doi: 10.1038/nrneurol.2015.243
Kim, J. A., Yoon, J. R., Lee, E. J., Lee, J. S., Kim, J. T., Kim, H. D., et al. (2016). Efficacy of the classic ketogenic and the modified Atkins diets in refractory childhood epilepsy. Epilepsia 57, 51–58. doi: 10.1111/epi.13256
Koelsch, S. (2010). Towards a neural basis of music-evoked emotions. Trends Cogn. Sci. 14, 131–137. doi: 10.1016/j.tics.2010.01.002
Koelsch, S. (2014). Brain correlates of music-evoked emotions. Nat. Rev. Neurosci. 15, 170–180. doi: 10.1038/nrn3666
Kotwas, I., McGonigal, A., Bastien-Toniazzo, M., Bartolomei, F., and Micoulaud-Franchi, J. A. (2017). Stress regulation in drug-resistant epilepsy. Epilepsy Behav. 71(Pt A):39–50. doi: 10.1016/j.yebeh.2017.01.025
Leenen, L. A. M., Wijnen, B. F. M., de Kinderen, R. J. A., van Heugten, C. M., Evers, S., and Majoie, M. (2016). Are people with epilepsy using eHealth-tools? Epilepsy Behav. 64(Pt A), 268–272. doi: 10.1016/j.yebeh.2016.08.007
Le Marne, F. A., Butler, S., Beavis, E., Gill, D., and Bye, A. M. E. (2018). EpApp: Development and evaluation of a smartphone/tablet app for adolescents with epilepsy. J. Clin. Neurosci. 50, 214–220. doi: 10.1016/j.jocn.2018.01.065
Leubner, D., and Hinterberger, T. (2017). Reviewing the effectiveness of music interventions in treating depression. Front. Psychol. 8:1109. doi: 10.3389/fpsyg.2017.01109
Lin, L. C., Chiang, C. T., Lee, M. W., Mok, H. K., Yang, Y. H., Wu, H. C., et al. (2013). Parasympathetic activation is involved in reducing epileptiform discharges when listening to Mozart music. Clin. Neurophysiol. 124, 1528–1535. doi: 10.1016/j.clinph.2013.02.021
Lin, L. C., Juan, C. T., Chang, H. W., Chiang, C. T., Wei, R. C., Lee, M. W., et al. (2013). Mozart K.448 attenuates spontaneous absence seizure and related high-voltage rhythmic spike discharges in Long Evans rats. Epilepsy Res. 104, 234–240. doi: 10.1016/j.eplepsyres.2012.11.005
Lin, L. C., Lee, M. W., Wei, R. C., Mok, H. K., Wu, H. C., Tsai, C. L., et al. (2012). Mozart k.545 mimics mozart k.448 in reducing epileptiform discharges in epileptic children. Evid. Based Complement. Alternat. Med. 2012:607517. doi: 10.1155/2012/607517
Lin, L. C., Lee, M. W., Wei, R. C., Mok, H. K., and Yang, R. C. (2014a). Mozart K.448 listening decreased seizure recurrence and epileptiform discharges in children with first unprovoked seizures: a randomized controlled study. BMC Complement. Altern. Med. 14:17. doi: 10.1186/1472-6882-14-17
Lin, L. C., Lee, W. T., Wang, C. H., Chen, H. L., Wu, H. C., Tsai, C. L., et al. (2011a). Mozart K.448 acts as a potential add-on therapy in children with refractory epilepsy. Epilepsy Behav. 20, 490–493. doi: 10.1016/j.yebeh.2010.12.044
Lin, L. C., Lee, W. T., Wu, H. C., Tsai, C. L., Wei, R. C., Jong, Y. J., et al. (2010). Mozart K.448 and epileptiform discharges: effect of ratio of lower to higher harmonics. Epilepsy Res. 89, 238–245. doi: 10.1016/j.eplepsyres.2010.01.007
Lin, L. C., Lee, W. T., Wu, H. C., Tsai, C. L., Wei, R. C., Mok, H. K., et al. (2011b). The long-term effect of listening to Mozart K.448 decreases epileptiform discharges in children with epilepsy. Epilepsy Behav. 21, 420–424. doi: 10.1016/j.yebeh.2011.05.015
Lin, L. C., Ouyang, C. S., Chiang, C. T., Wu, H. C., and Yang, R. C. (2014b). Early evaluation of the therapeutic effectiveness in children with epilepsy by quantitative EEG: a model of Mozart K.448 listening–a preliminary study. Epilepsy Res. 108, 1417–1426. doi: 10.1016/j.eplepsyres.2014.06.020
Liu, X., Wang, R., Zhou, D., and Hong, Z. (2015). Feasibility and acceptability of smartphone applications for seizure self-management in China: questionnaire study among people with epilepsy. Epilepsy Behav. 55, 57–61. doi: 10.1016/j.yebeh.2015.11.024
Liu, X., Wang, R., Zhou, D., and Hong, Z. (2016). Smartphone applications for seizure care and management in children and adolescents with epilepsy: feasibility and acceptability assessment among caregivers in China. Epilepsy Res. 127, 1–5. doi: 10.1016/j.eplepsyres.2016.08.002
Lundgren, T., Dahl, J., Melin, L., and Kies, B. (2006). Evaluation of acceptance and commitment therapy for drug refractory epilepsy: a randomized controlled trial in South Africa–a pilot study. Epilepsia 47, 2173–2179. doi: 10.1111/j.1528-1167.2006.00892.x
Lundgren, T., Dahl, J., Yardi, N., and Melin, L. (2008). Acceptance and commitment therapy and yoga for drug-refractory epilepsy: a randomized controlled trial. Epilepsy Behav. 13, 102–108. doi: 10.1016/j.yebeh.2008.02.009
Maguire, J., and Salpekar, J. A. (2013). Stress, seizures, and hypothalamic-pituitary-adrenal axis targets for the treatment of epilepsy. Epilepsy Behav. 26, 352–362. doi: 10.1016/j.yebeh.2012.09.040
Malek, N., Heath, C. A., and Greene, J. (2017). A review of medication adherence in people with epilepsy. Acta Neurol. Scand. 135, 507–515. doi: 10.1111/ane.12703
Mantani, A., Kato, T., Furukawa, T. A., Horikoshi, M., Imai, H., Hiroe, T., et al. (2017). Smartphone cognitive behavioral therapy as an adjunct to pharmacotherapy for refractory depression: randomized controlled trial. J. Med. Internet Res. 19:e373. doi: 10.2196/jmir.8602
Martin, K., Jackson, C. F., Levy, R. G., and Cooper, P. N. (2016). Ketogenic diet and other dietary treatments for epilepsy. Cochrane Database Syst. Rev. 2:CD001903. doi: 10.1002/14651858.CD001903.pub3
May, T. W., and Pfafflin, M. (2002). The efficacy of an educational treatment program for patients with epilepsy (MOSES): results of a controlled, randomized study. Modular Service Package Epilepsy. Epilepsia 43, 539–549. doi: 10.1046/j.1528-1157.2002.23801.x
McKennon, S., Levitt, S. E., and Bulaj, G. (2017). Commentary: a breathing-based meditation intervention for patients with major depressive disorder following inadequate response to antidepressants: a randomized pilot study. Front. Med. (Lausanne). 4:37. doi: 10.3389/fmed.2017.00037
McLaughlin, D. P., and McFarland, K. (2011). A randomized trial of a group based cognitive behavior therapy program for older adults with epilepsy: the impact on seizure frequency, depression and psychosocial well-being. J. Behav. Med. 34, 201–207. doi: 10.1007/s10865-010-9299-z
McSherry, T., Atterbury, M., Gartner, S., Helmold, E., Searles, D. M., and Schulman, C. (2017). Randomized, crossover study of immersive virtual reality to decrease opioid use during painful wound care procedures in adults. J. Burn. Care Res. 39, 278–285. doi: 10.1097/BCR.0000000000000589
Meng, B., Zhu, S., Li, S., Zeng, Q., and Mei, B. (2009). Global view of the mechanisms of improved learning and memory capability in mice with music-exposure by microarray. Brain Res. Bull. 80, 36–44. doi: 10.1016/j.brainresbull.2009.05.020
Merry, S. N., Stasiak, K., Shepherd, M., Frampton, C., Fleming, T., and Lucassen, M. F. (2012). The effectiveness of SPARX, a computerised self help intervention for adolescents seeking help for depression: randomised controlled non-inferiority trial. BMJ 344:e2598. doi: 10.1136/bmj.e2598
Michaelis, R., Tang, V., Wagner, J. L., Modi, A. C., LaFrance, W. C. Jr., Goldstein, L. H., et al. (2017). Psychological treatments for people with epilepsy. Cochrane Database Syst. Rev. 10:CD012081. doi: 10.1002/14651858.CD012081.pub2
Mittan, R. J. (2009). Psychosocial treatment programs in epilepsy: a review. Epilepsy Behav. 16, 371–380. doi: 10.1016/j.yebeh.2009.08.031
Modi, A. C., Schmidt, M., Smith, A. W., Turnier, L., Glaser, N., and Wade, S. L. (2017). Development of a web-based executive functioning intervention for adolescents with epilepsy: the epilepsy journey. Epilepsy Behav. 72, 114–121. doi: 10.1016/j.yebeh.2017.04.009
O' Rourke, G., and O' Brien, J. J. (2017). Identifying the barriers to antiepileptic drug adherence among adults with epilepsy. Seizure 45:160–168. doi: 10.1016/j.seizure.2016.12.006
Page, R., Shankar, R., McLean, B. N., Hanna, J., and Newman, C. (2018). Digital care in epilepsy: a conceptual framework for technological therapies. Front. Neurol. 9:99. doi: 10.3389/fneur.2018.00099
Pandher, P. S., and Bhullar, K. K. (2014). Smartphone applications for seizure management. Health Inform. J. 22, 209–220. doi: 10.1177/1460458214540906
Park, S., Lee, D. H., Kim, S. W., and Roh, Y. H. (2017). Prognostic analysis of patients with epilepsy according to time of relapse after withdrawal of antiepileptic drugs following four seizure-free years. Epilepsia 58, 60–67. doi: 10.1111/epi.13624
Pirbaglou, M., Katz, J., de Souza, R. J., Stearns, J. C., Motamed, M., and Ritvo, P. (2016). Probiotic supplementation can positively affect anxiety and depressive symptoms: a systematic review of randomized controlled trials. Nutr. Res. 36, 889–898. doi: 10.1016/j.nutres.2016.06.009
Pollock, A., Baer, G., Campbell, P., Choo, P. L., Forster, A., Morris, J., et al. (2014a). Physical rehabilitation approaches for the recovery of function and mobility following stroke. Cochrane Database Syst. Rev. CD001920. doi: 10.1002/14651858.CD001920.pub3
Pollock, A., Farmer, S. E., Brady, M. C., Langhorne, P., Mead, G. E., Mehrholz, J., et al. (2014b). Interventions for improving upper limb function after stroke. Cochrane Database Syst. Rev. CD010820. doi: 10.1002/14651858.CD010820.pub2
Qaseem, A., Wilt, T. J., McLean, R. M., Forciea, M. A., and Clinical Guidelines Committee of the American College of, P. (2017). Noninvasive treatments for acute, subacute, and chronic low back pain: a clinical practice guideline from the american college of physicians. Ann. Intern. Med. 166, 514–530. doi: 10.7326/M16-2367
Ramirez, R., Palencia-Lefler, M., Giraldo, S., and Vamvakousis, Z. (2015). Musical neurofeedback for treating depression in elderly people. Front. Neurosci. 9:354. doi: 10.3389/fnins.2015.00354
Roeder, L., Costello, J. T., Smith, S. S., Stewart, I. B., and Kerr, G. K. (2015). Effects of resistance training on measures of muscular strength in people with parkinson's disease: a systematic review and meta-analysis. PLoS ONE 10:e0132135. doi: 10.1371/journal.pone.0132135
Roepke, A. M., Jaffee, S. R., Riffle, O. M., McGonigal, J., Broome, R., and Maxwell, B. (2015). Randomized controlled trial of superbetter, a smartphone-based/internet-based self-help tool to reduce depressive symptoms. Games Health J. 4, 235–246. doi: 10.1089/g4h.2014.0046
Salimpoor, V. N., Benovoy, M., Larcher, K., Dagher, A., and Zatorre, R. J. (2011). Anatomically distinct dopamine release during anticipation and experience of peak emotion to music. Nat. Neurosci. 14, 257–262. doi: 10.1038/nn.2726
Schriewer, K., and Bulaj, G. (2016). Music streaming services as adjunct therapies for depression, anxiety, and bipolar symptoms: convergence of digital technologies, mobile apps, emotions, and global mental health. Front. Public Health 4:217. doi: 10.3389/fpubh.2016.00217
Seidl, S. E., Santiago, J. A., Bilyk, H., and Potashkin, J. A. (2014). The emerging role of nutrition in Parkinson's disease. Front. Aging Neurosci. 6:36. doi: 10.3389/fnagi.2014.00036
Sihvonen, A. J., Särkämö, T., Leo, V., Tervaniemi, M., Altenmüller, E., and Soinila, S. (2017). Music-based interventions in neurological rehabilitation. Lancet Neurol. 16, 648–660. doi: 10.1016/S1474-4422(17)30168-0
Spector, S., Cull, C., and Goldstein, L. H. (2000). Seizure precipitants and perceived self-control of seizures in adults with poorly-controlled epilepsy. Epilepsy Res. 38, 207–216. doi: 10.1016/S0920-1211(99)00093-5
Spector, S., Cull, C., and Goldstein, L. H. (2001). High and low perceived self-control of epileptic seizures. Epilepsia 42, 556–564. doi: 10.1046/j.1528-1157.2001.09800.x
Spector, S., Tranah, A., Cull, C., and Goldstein, L. H. (1999). Reduction in seizure frequency following a short-term group intervention for adults with epilepsy. Seizure 8, 297–303. doi: 10.1053/seiz.1999.0292
Tang, F., Hartz, A. M. S., and Bauer, B. (2017). Drug-resistant epilepsy: multiple hypotheses, few answers. Front Neurol. 8:301. doi: 10.3389/fneur.2017.00301
Tang, V., Poon, W. S., and Kwan, P. (2015). Mindfulness-based therapy for drug-resistant epilepsy: an assessor-blinded randomized trial. Neurology 85, 1100–1107. doi: 10.1212/WNL.0000000000001967
Tashjian, V. C., Mosadeghi, S., Howard, A. R., Lopez, M., Dupuy, T., Reid, M., et al. (2017). Virtual reality for management of pain in hospitalized patients: results of a controlled trial. JMIR Ment. Health 4:e9. doi: 10.2196/mental.7387
Tasset, I., Quero, I., García-Mayórgaz, Á. D., del Río, M. C., Túnez, I., and Montilla, P. (2012). Changes caused by haloperidol are blocked by music in Wistar rat. J. Physiol. Biochem. 68, 175–179. doi: 10.1007/s13105-011-0129-8
Tieffenberg, J. A., Wood, E. I., Alonso, A., Tossutti, M. S., and Vicente, M. F. (2000). A randomized field trial of ACINDES: a child-centered training model for children with chronic illnesses (asthma and epilepsy). J. Urban Health 77, 280–297. doi: 10.1007/BF02390539
Toups, M., Carmody, T., Greer, T., Rethorst, C., Grannemann, B., and Trivedi, M. H. (2017). Exercise is an effective treatment for positive valence symptoms in major depression. J. Affect. Disord. 209, 188–194. doi: 10.1016/j.jad.2016.08.058
van Andel, J., Westerhuis, W., Zijlmans, M., Fischer, K., and Leijten, F. S. (2011). Coping style and health-related quality of life in caregivers of epilepsy patients. J. Neurol. 258, 1788–1794. doi: 10.1007/s00415-011-6013-1
Walker, E. R., Bamps, Y., Burdett, A., Rothkopf, J., and Diiorio, C. (2012). Social support for self-management behaviors among people with epilepsy: a content analysis of the WebEase program. Epilepsy Behav. 23, 285–290. doi: 10.1016/j.yebeh.2012.01.006
Walker, E. R., Engelhard, G., Barmon, C., McGee, R. E., Sterk, C. E., Diiorio, C., et al. (2014). A mixed methods analysis of support for self-management behaviors: perspectives of people with epilepsy and their support providers. Epilepsy Behav. 31:152–159. doi: 10.1016/j.yebeh.2013.11.023
Wulsin, A. C., Solomon, M. B., Privitera, M. D., Danzer, S. C., and Herman, J. P. (2016). Hypothalamic-pituitary-adrenocortical axis dysfunction in epilepsy. Physiol. Behav. 166, 22–31. doi: 10.1016/j.physbeh.2016.05.015
Xing, Y., Chen, W., Wang, Y., Jing, W., Gao, S., Guo, D., et al. (2016a). Music exposure improves spatial cognition by enhancing the BDNF level of dorsal hippocampal subregions in the developing rats. Brain Res. Bull. 121, 131–137 doi: 10.1016/j.brainresbull.2016.01.009
Xing, Y., Qin, Y., Jing, W., Zhang, Y., Wang, Y., Guo, D., et al. (2016b). Exposure to Mozart music reduces cognitive impairment in pilocarpine-induced status epilepticus rats. Cogn. Neurodyn. 10, 23–30. doi: 10.1007/s11571-015-9361-1
Keywords: digital medicine, eHealth, mHealth, refractory epilepsy, antiepileptic drugs, anxiety, antiseizure, Mozart
Citation: Afra P, Bruggers CS, Sweney M, Fagatele L, Alavi F, Greenwald M, Huntsman M, Nguyen K, Jones JK, Shantz D and Bulaj G (2018) Mobile Software as a Medical Device (SaMD) for the Treatment of Epilepsy: Development of Digital Therapeutics Comprising Behavioral and Music-Based Interventions for Neurological Disorders. Front. Hum. Neurosci. 12:171. doi: 10.3389/fnhum.2018.00171
Received: 24 January 2018; Accepted: 12 April 2018;
Published: 01 May 2018.
Edited by:
Mikhail Lebedev, Duke University, United StatesReviewed by:
Omid Kavehei, University of Sydney, AustraliaSheffali Gulati, All India Institute of Medical Sciences, India
Copyright © 2018 Afra, Bruggers, Sweney, Fagatele, Alavi, Greenwald, Huntsman, Nguyen, Jones, Shantz and Bulaj. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pegah Afra, pegah.afra@hsc.utah.edu
Grzegorz Bulaj, bulaj@pharm.utah.edu
†Present Address: Michael Greenwald, Westminster College, Salt Lake City, UT, United States
Merodean Huntsman, Tulane University, New Orleans, LA, United States
Jeremiah K. Jones, Stretto Consulting, St. George, UT, United States
 Pegah Afra
Pegah Afra Carol S. Bruggers
Carol S. Bruggers Matthew Sweney1,3
Matthew Sweney1,3  Fareeha Alavi
Fareeha Alavi Jeremiah K. Jones
Jeremiah K. Jones Grzegorz Bulaj
Grzegorz Bulaj