Abstract
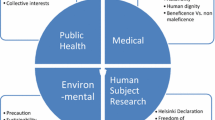
This paper, guided by the UNESCO Universal Declaration on Bioethics and Human Rights, assumes that regulators should aim to support the development of nanomedicine while, at the same time, putting in place whatever limits or safeguards are indicated by ethical considerations. Relative to this regulatory objective, it is argued that, notwithstanding the importance of precaution (characteristically, concerning health, safety, and the environment), ethical reflection needs to go both broader and deeper. It is suggested that, by attending to the basic matrix of ethical debate and the “bioethical triangle” through which the matrix is currently articulated, the breadth, depth, and conflictual plurality of ethical concerns about nanomedicine will be clarified. In this light, the conventional thinking about precaution is revisited and concerns about human dignity and informed consent (under conditions of extreme uncertainty) are analysed. The paper concludes that, once the range of ethical pluralism is grasped, the extent of the challenge facing regulators will be more clearly appreciated.
Similar content being viewed by others
Notes
Compare Spinello [52]. Spinello employs three ethical approaches (utilitarian, contractarian rights-based, and Kantian duty-based), in conjunction with a post-Lessig [37] range of regulatory options, to review four key issues: namely, freedom of on-line expression, intellectual property in cyberspace, Internet privacy, and security.
For dwarf-throwing, see Conseil d’Etat (October 27, 1995) req. nos. 136–727 (Commune de Morsang-sur-Orge) and 143–578 (Ville d’Aix-en-Provence); and, for the Laserdrome, see Omega Spielhallen-und Automatenaufstellungs-GmbH v Oberbürgermeisterin der Bundesstadt Bonn (Case C-36/02, 14 October, 2004); OJ C 300, 04.12.2004 p.3.
But, nb van den Daele [59] for a caution concerning the way in which the precautionary principle can be deployed disingenuously in defence of cultural conservativism.
There are, it should be said, some promising near-term applications. For example, the so-called Trojan Horse (a nano-based vehicle for anti-cancer drug delivery) is in phase I clinical trial at the University of Michigan: see Wilson [61] at 706. On other hand, we should not assume that research and innovation will translate into exploitation, use and application: see Edgerton [18].
See, the European Group on Ethics in Science and New Technologies to the European Commission ([56] pp. 42–43) and Appendix I.
For the background, see Jean et al. [33].
On these second and third considerations, see Bauer [3], esp at 8–11. See, too, the Nuffield Council on Bioethics [43] at 8: “it is always possible, in principle, to distinguish between the two distinct questions of ‘how bad?’ and ‘how likely?’ That is, we can and should separate the reasons for regarding an outcome as evil from the likelihood of its occurrence.”
It is estimated that there are already more than 200 nanotechnology-based products in the consumer marketplace, see Wardak and Gorman [60].
See the important discussion of this phenomenon in Leader [35].
Compare Sheremata [51].
This is a distinction that I discuss more fully in Brownsword [12] (in press).
The relationship between a community of rights and a culture of trust is not entirely straightforward: see, O’Neill [44].
References
Ashcroft R, Campbell AV, Capps B (2007) Ethical aspects of developments in neuroscience and drug addiction. In: Nutt D, Robbins TW, Stimson GW, Ince M, Jackson A (eds) Drugs and the Future. Academic Press, London, pp 439–465
Barnett J, Carr A, Clift R (2006) Going public: risk, trust and public understanding of nanotechnologies. In: Hunt G, Mehta M (eds) Nanotechnology: risk, ethics and law. Earthscan, London, pp 196–212
Bauer M (1995) Resistance to new technology and its effects on nuclear power, information technology and biotechnology. In: Bauer M (ed) Resistance to new technology. Cambridge, University Press: Cambridge, pp 1–41
Bauer MW, Gaskell G (eds) (2002) Biotechnology—the making of a global controversy. Cambridge University Press, Cambridge
Best R, Khushf G (2006) The social conditions for nanomedicine: disruption, systems, and lock-in. J Law Med Ethics 34:733–740
Beyleveld D, Brownsword R (2001) Human dignity in bioethics and biolaw. Oxford University Press, Oxford
Beyleveld D, Brownsword R (2007) Consent in the law. Hart, Oxford
Brownsword R (2004a) Three bioethical approaches: a triangle to be squared. Paper presented at international conference on the patentability of biotechnology organised by the Sasakawa Peace Foundation, Tokyo
Brownsword R (2004b) Regulating human genetics: new dilemmas for a new millennium. Med Law Rev 12:14–39
Brownsword R (2005a) Stem cells and cloning: where the regulatory consensus fails. New Engl Law Rev 39:535–571
Brownsword R (2005b) Human rights—what hope? Human dignity—what scope? In: Gunning J, Holm S (eds) Ethics, law and society, vol 1. Ashgate, Aldershot, pp 189–209
Brownsword R (2008) Rights, regulation and the technological revolution. Oxford University Press, Oxford (in press)
Butler S (1985) Erewhon (first published 1872). Penguin Books, London
COMEST (2007) Nanotechnology and ethics: policies and actions. UNESCO, Paris
Court EB, Salamanca-Buentello F, Singer PA, Daar AS (2007) Nanotechnology and the developing world. In: ten Have H (ed) Nanotechnologies, ethics and politics. UNESCO, Paris, pp 155–180
Davies JC (2006) Managing the effects of nanotechnology. Washington: Woodrow Wilson International Center for Scholars. http://www.wilsoncenter.org/events/docs/Effectsnanotechfinal.pdf. Cited August 30, 2007
Dworkin RM (1993) Life’s dominion. Harper Collins, London
Edgerton D (2006) The shock of the old: technology and global history since 1900. Profile Books, London
Einsiedel EF, Goldberg L (2006) Dwarfing the social? Nanotechnology lessons from the biotechnology front. In: Hunt G, Mehta M (eds) Nanotechnology: risk, ethics and law. Earthscan, London, pp 213–221
European Technology Platform (2006) Nanomedicine: nanotechnology for health
Evans D (2007) Ethics, nanotechnology and health. In: ten Have H (ed) Nanotechnologies, ethics and politics. UNESCO, Paris, pp 125–153
Frean A, Foster P (2007) Cheating students turn to ‘smart drugs’ for edge in exams. The Times June 23:16–17
Galligan DJ (2007) Citizens’ rights and participation in the regulation of biotechnology. In: Francioni F (ed) Biotechnologies and international human rights. Hart, Oxford, pp 335–359
Gordijn B (2006) Converging NBIC technologies for improving human performance: a critical assessment of the novelty and prospects of the project. J Law Med Ethics 34:726–732
Gordijn B (2007) Ethical issues in nanomedicine. In: ten Have H (ed) Nanotechnologies, ethics and politics. UNESCO, Paris, pp 99–123
Heyvaert V (2006a) Guidance without constraint: assessing the impact of the precautionary principle on the european community’s chemicals policy. The Yearbook of European Environmental Law 6:27–60
Heyvaert V (2006b) Facing the consequences of the precautionary principle in european community law. Eur Law Rev 31:185–206
House of Commons Science and Technology Select Committee (2007) Government proposals for the regulation of hybrid and chimera embryos. Fifth Report of Session 2006–07, HC 272-I)
Howard CV, Ikah DSK (2006) Nanotechnology and nanoparticle toxicity: a case for precaution. In: Hunt G, Mehta M (eds) Nanotechnology: risk, ethics and law. Earthscan, London, pp 154–166
Hunt G (2006) The global ethics of nanotechnology. In: Hunt G, Mehta M (eds) Nanotechnology: risk, ethics and law. Earthscan, London, pp 183–195
Hunt G, Mehta M (eds) (2006) Nanotechnology: risk, ethics and law. London: Earthscan
Jasanoff S (1995) Product, process, or programme: three cultures and the regulation of biotechnology. In: Bauer M (ed) Resistance to new technology. Cambridge University Press, Cambridge, pp 311–331
Jean MS, Deleury E, Duquet D (2007) Early assessment and policy-making. In: ten Have H (ed) Nanotechnologies, ethics and politics. UNESCO, Paris, pp 205–219
Kass LR (2002) Life, liberty and the defense of dignity. Encounter Books, San Francisco
Leader S (2004) Collateralism. In: Brownsword R (ed) Human rights. Hart, Oxford, pp 53–67
Lee R, Stokes E (2005) Ecological modernisation and the precautionary principle. In: Gunning J, Holm S (eds) Ethics, law and society, vol 1. Ashgate, Aldershot, pp 103–113
Lessig L (1999) Code and other laws of cyberspace. Basic Books, New York
Levy N (2007) Neuroethics. Cambridge University Press, Cambridge
Manson N, O’Neill O (2007) Rethinking informed consent in bioethics. Cambridge University Press, Cambridge
Marchant GE, Sylvester DJ (2006) Transnational models for regulation of nanotechnology. J Law Med Ethics 34:714–725
Mehta MD (2006) From biotechnology to nanotechnology: what can we learn from earlier technologies? In: Hunt G, Mehta M (eds) Nanotechnology: risk, ethics and law. Earthscan, London, pp 121–129
Michelman FI (2003) Constitutional legitimation for political acts. Mod Law Rev 66:1–15
Nuffield Council on Bioethics (1999) Genetically modified crops: the ethical and social issues. Nuffield Council on Bioethics, London
O’Neill O (2002) Autonomy and trust in bioethics. Cambridge University Press, Cambridge
O’Neill O (2003) Some limits of informed consent. J Med Ethics 29:4–7
Quebec Commission on the Ethics of Science and Technology (2006) Ethics and nanotechnologies: a basis for action
President’s Council on Bioethics (2003) Beyond therapy. Dana, New York
Room R (2007) Social policy and psychoactive substances. In: Nutt D, Robbins TW, Stimson GW, Ince M, Jackson A (eds) Drugs and the future. Academic, London, pp 337–358
Sandler R, Kay WD (2006) The national nanotechnology initiative and the social good. J Law Med Ethics, 34:675–681
Schummer J (2007) Identifying ethical issues of nanotechnologies. In: ten Have H (ed) Nanotechnologies, ethics and politics. UNESCO, Paris, pp 79–98
Sheremata L (2006) Nanotechnologies and the ethical conduct of research involving human subjects. In: Hunt G, Mehta M (eds) Nanotechnology: risk, ethics and law. Earthscan, London, pp 247–258
Spinello RA (2006) Cyberethics: morality and law in cyberspace, 3rd ed. Jones and Bartlett, Sudbury, MA
Standage T (ed) (2005) The future of technology. Profile Books, London
Sunstein C (2005) Laws of fear. Cambridge University Press, Cambridge
The Council for Science and Technology (2007) Nanosciences and nanotechnologies: a review of government’s progress on its policy commitments. The Council for Science and Technology, London
The European Group on Ethics in Science and New Technologies to the European Commission (2007) Opinion on the ethical aspects of nanomedicine. Opinion no. 21
The Royal Society and the Royal Academy of Engineering (2004) Nanoscience and nanotechnologies: opportunities and uncertainties. The Royal Society, London (RS Policy document 19/04)
UNESCO (2005) UNESCO universal declaration on bioethics and human rights
van den Daele W (2007) Legal framework and political strategy in dealing with the risks of new technology: the two faces of the precautionary principle. In: Somsen H (ed) The regulatory challenge of biotechnology. Edward Elgar, Cheltenham, pp 118–138
Wardak A, Gorman ME (2006) Using trading zones and life cycle analysis to understand nanotechnology regulation. J Law Med Ethics 34:695–703
Wilson RF (2006) Nanotechnology: the challenge of regulating known unknowns. J Law Med Ethics 34:704–713
Winickoff D, Jasanoff S, Busch L, Grove-White R, Wynne B (2005) Adjudicating the GM food wars: Science, risk, and democracy in world trade law. Yale J Int Law 30:81–123
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Brownsword, R. Regulating Nanomedicine—The Smallest of Our Concerns?. Nanoethics 2, 73–86 (2008). https://doi.org/10.1007/s11569-008-0030-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11569-008-0030-2