- 1Medical Psychological Center, The Second Xiangya Hospital, Central South University, Changsha, China
- 2Preschool Education Department, Changsha Normal University, Changsha, China
- 3Department of Psychiatry, The First Affiliated Hospital of Soochow University, Soochow University, Suzhou, China
Purpose: Although efforts have been made to identify neurobiological characteristic of major depressive disorder (MDD) in recent years, trait- and state-related biological characteristics of MDD still remains unclear. Using functional magnetic resonance imaging (fMRI), the aim of this study was to explore whether altered spontaneous neural activities in MDD are trait- or state- related.
Materials and Methods: Resting-state fMRI data were analyzed for 72 current MDD (cMDD) patients (first-episode, medication-naïve), 49 remitted MDD (rMDD) patients, and 78 age- and sex- matched healthy control (HC) subjects. The values of amplitude of low-frequency fluctuation (ALFF) were compared between groups.
Results: Compared with the cMDD group, the rMDD group had increased ALFF values in the left middle occipital gyrus, left middle temporal gyrus and right cerebellum anterior lobe. Besides, compared with the HC group, the cMDD group had decreased ALFF values in the left middle occipital gyrus. Further analysis explored that the mean ALFF values in the left middle occipital gyrus, left middle temporal gyrus and right cerebellum anterior lobe were correlated positively with BDI scores in rMDD patients.
Conclusion: Abnormal activity in the left middle occipital gyrus, left middle temporal gyrus and right cerebellum anterior lobe may be state-specific in current (first-episode, medication-naïve) and remitted (medication-naïve) depression patients. Furthermore, the state-related compensatory effect was found in these brain areas.
Introduction
Major depressive disorder (MDD) is a high-recurrence psychiatric condition. which more than half of patients seen for first-episode MDD in China experience a recurrence of MDD symptoms within 5 years after the initial depression onset (Ji et al., 2001). Moreover, clinical factors, including the number of previous episodes and subclinical residual symptoms, appear to be the most important predictors of recurrence (Hardeveld et al., 2010). Therefore, distinguishing the correlation between altered brain activity and different clinical states may lead to a better understanding of the neurobiological mechanisms underlying MDD pathogenesis and recurrence, which, in turn, may improve clinical diagnosis and prognostic evaluation of MDD.
Some neuroimaging studies, primarily using task-related functional magnetic resonance imaging (fMRI) and structural magnetic resonance imaging (sMRI) methods, have examined brain activation and structural changes in different clinical states, including current MDD (cMDD) and remitted MDD (rMDD). These researches have explored several abnormal alterations of brain region in patients, which could be trait-related or state-related markers of depression. However, a number of brain regions, including regions within the orbitofrontal cortex, insular cortex, posterior cingulate cortex (PCC), amygdala, hippocampus, and prefrontal cortex, have been dually implicated as potential trait-related and state-related biomarkers of depression (Caetano et al., 2004; Wang et al., 2008; Lorenzetti et al., 2010; van Eijndhoven et al., 2013; Liu et al., 2014; Ming Q. et al., 2017). Inconsistency across studies might be partially related to different task paradigms or analytical methods, including stress task or source recollection paradigm, cortical thickness or brain region volume analysis.
Resting-state fMRI studies, wherein subjects are not performing an explicit task during the scan, can complement task fMRI studies (Su et al., 2010; Biswal, 2012). Resting-state data can be analyzed by multifarious approaches, such as seed-based approaches, independent component analysis, graph methods, clustering algorithms, neural networks, and pattern classifier (Lee et al., 2013; Dong et al., 2018). In recent years, a resting-state fMRI analytical method named amplitude of low-frequency fluctuation (ALFF) was developed to assess the spontaneous low frequency (0.01–0.08 Hz) fluctuations (LFF) in the BOLD fMRI signal at rest, which could reflect the intensity of brain regional spontaneous neural activity (Zang et al., 2007). Besides, some articles indicated that the ALFF was associated with the neuronal glucose metabolism and local field potential activity (Logothetis et al., 2001; Tomasi et al., 2013). Therefore ALFF analysis has been widely used to explore potentially related cerebral biomarkers in mental disorders, which was shown to be a reliable and sensitive approach (Zhang et al., 2014; Fan et al., 2017).
To our knowledge, only one resting-state fMRI study investigating state- and trait-related functional alterations in cMDD and rMDD have been published. Jing reported that abnormal activity of the putamen may be a potential trait-related marker of MDD (Jing et al., 2013). However, Jing’s study included only female patients and did not exclude the effects of comorbidities, treatment condition, or the number of prior episodes. Some depression researches indicated that the comorbidities, treatment condition, and the number of prior episodes would make a difference in the activities of brain areas, such as cingulate cortex, prefrontal lobe, striatum, temporal lobe and insula (Schaefer et al., 2006; Takami et al., 2007; Waugh et al., 2012). Thus the effects of comorbidities, treatment condition, the number of prior episodes should be considered in the depression study.
Notably, Mayberg’s classical neurobiological model implied that the MDD patient has fronto-limbic dysfunctions, including the prefrontal cortex, cingulate cortex, amygdala and striatum, which would account for the dysregulation of the affective and cognitive behavior in patients (Mayberg, 1997, 2003). Moreover, some meta-analysis articles of resting-state fMRI suggested that the dysfunctions of the fronto-limbic circuit, default mode network (DMN) and cerebellum, which play an important role in the cognitive processing and affective regulation, would be the biomarkers of drug-naive MDD patients (Xue et al., 2016; Ming Z.M. et al., 2017).
The purpose of this study was to investigate MDD state-related and trait-related neuroimaging alterations of spontaneous neural activity by resting-state fMRI. To exclude the potential influence of comorbidities, treatment condition, and the number of prior episodes, we enrolled a large sample, including 72 first-episode, medication-naive MDD patients (cMDD), 49 remitted MDD patients (rMDD), and 78 age- and sex- matched healthy controls (HCs). The participants were subjected to resting-state fMRI with ALFF analysis. In the context of classical neurobiological model and the studies mentioned above, we hypothesized that the state-related or trait-related characteristic of MDD would be explored in the brain areas of fronto-limbic circuit, default mode network and cerebellum.
Materials and Methods
Participants
The cMDD and rMDD participants were recruited from the psychology clinic at Second Xiangya Hospital affiliated with Central South University in Changsha, Hunan, China. With advertisements and posters, age-, sex-matched HCs were recruited from two colleges and a local community in Changsha. The sample of all the depression patents and normal people was registered from 2014 to 2017.
The clinical states of the patients, including cMDD and rMDD groups, were evaluated independently by two psychiatrists using the Structured Clinical Interview for DSM-IV-TR Axis I Disorders-Patient Edition (First et al., 2002) and 17-item Hamilton Depression Rating Scale (Hamilton, 1960). At the same time, demographic data and clinical variables information were collected by interview. Patients, who met the DSM-IV-TR criteria for MDD and were in their first MDD, were included in the cMDD group. Inclusion criteria for the rMDD patients were as follows: having at least one episode of MDD in the past 10 years; not meeting the DSM-IV-TR criteria for MDD more than 30 days before the scan; a 17-item HAM-D score ≤7 on the scan day. In this study the remitted MDD was not described as clinical outcome of recovery but a remitted state, which was not shown currently presenting symptoms of MDD. Moreover, this criterion has been used in previous studies of remitted depression (Goulden et al., 2012). The HC group was required to have no history of any prior DSM-IV-TR Axis I disorder. The exclusion criteria for all three groups were: a history of alcohol/substance abuse; use of antidepressants or undergoing psychotherapy or psychotropic medications; having other major psychiatric disorder; a neurological disorder diagnosis; structural brain abnormalities; and any MRI contraindication.
All participants were informed of the study’s purpose and signed informed consent forms. This study was approved by the Ethics Committee of the Second Xiangya Hospital of Central South University.
Psychological Measures
All participants filled out a Beck Depression Inventory-II (Beck et al., 1996) composed of 21 self-report items, which proved to be a validated depressive symptoms scale. This multiple-choice inventory could evaluate the MDD symptoms including irritability, feelings of guilt, suicidal ideation, fatigue, and weight loss (Ayloo et al., 2015). In our current sample, Cronbach’s alphas for cMDD, rMDD, and HC groups were 0.850, 0.858, and 0.823, respectively.
Image Acquisition
Magnetic resonance imaging (MRI) scans were performed on a 3.0-T Siemens Magnetom Skyra scanner (Siemens Healthineers, Erlangen, Germany). During scanning, all participants were instructed to remain motionless with their eyes closed, and to think of nothing in particular but to not fall asleep. To reduce patient head movements and noise, the subjects were fit with foam pads about the head and earplugs. The acquisition parameters were as follows: 32 axial slices, 4-mm slice thickness with a 1-mm gap, 2000-ms repetition time, 30-ms echo time, 80° flip angle, 256 × 256-mm field of view, 64 × 64 data matrix. Three-dimensional T1-weighted magnetization-prepared rapid gradient echo sagittal images were also acquired with the following parameters: 176 axial slices with no gap, 1900-ms repetition time, 2.01-ms echo time, 1-mm3 voxel size, 9° flip angle, 256 × 256-mm field of view, 256 × 256 data matrix.
Data Processing
Resting-state fMRI data preprocessing was conducted in Data Processing Assistant for Resting-State fMRI software (DPARSF V2.3, Yan and Zang, 20101). The first 10 volumes of the functional time series were removed to ensure stable magnetization and adaptation of participants to scanning noise. Subsequently, slice timing and head motion correction were performed. Data were discarded from 30 subjects (10 cMDD, 9 rMDD, and 4 HC) due to translation >2 mm in any direction or rotation >2° around any axis in any of six head motion parameters. Besides, regression of the Friston 24 motion parameters was conducted to control the potential influence of head motion (Satterthwaite et al., 2013). After the correction mentioned above, the EPI template of standard Montreal Neurological Institute (MNI) was used for spatial normalization with a resampling voxel size of 3 × 3 × 3 mm3. The preprocessing image was spatially smoothed with an 8 × 8 × 8 mm full width at half-maximum Gaussian kernel.
To discard biases from low-frequency drift and high-frequency noise, detrending and band-pass (0.01–0.08 Hz) filtering were conducted. After that, the time series of each voxel was converted into the frequency domain, of which power spectrum was calculated. The square root was calculated at each frequency of the power spectrum. Finally, the average square root was then obtained at each voxel across the frequency range of 0.01–0.08 Hz, which was obtained to take as ALFF to reflect absolute intensity of brain spontaneous neural activity (Zang et al., 2007).
After the data processing, the final analysis included 72 medication-naive, first-episode cMDD patients (39 females), 49 rMDD patients (26 females), and 78 HC subjects (43 females).
ALFF Data Statistical Analysis
Amplitude of low-frequency fluctuations maps were processed using the Resting-State fMRI Data Analysis Toolkit (REST V1.8, Song et al., 2011; see text footnote1). These maps were then exported to SPM12 (Wellcome Trust Center for Neuroimaging, London, United Kingdom2) for statistical analyses. An analysis of covariance (ANCOVA) was used to detect between group differences on ALFF maps, including levels of education as a covariate of no interest. An initial voxelwise threshold of p < 0.001 uncorrected was used, to which a clusterwise correction (FDR) for multiple comparison was applied.
Independently of the result, the map from the uncorrected main effect of group from the ANCOVA was used to mask the exploratory between group comparisons among the three different groups (Henson et al., 2002; Fan et al., 2017). For these exploratory analyses, an initial voxelwise threshold of p < 0.001 uncorrected was used, to which a clusterwise correction for multiple comparison (FDR) was applied. An additional Bonferroni correction for multiple comparisons was applied on the cluster p-value to account for the number of tests performed (6 comparisons: cMDD > rMDD, cMDD < rMDD, cMDD > HC, cMDD < HC, rMDD > HC, rMDD < HC). A cluster with a p-value of 0.008 or below would therefore be significant (p = 0.05/6 = 0.008).
To further examining the connection between the abnormal regional brain activity and depression symptom severity, correlation analyses were conducted between psychometric (HAM-D and BDI) scores and mean ALFF values obtained in brain regions with abnormal activity in the cMDD and rMDD groups, separately.
Results
Cohort Characteristics
The demographic and clinical data are summarized in Table 1. The groups’ characteristics did not differ in terms of sex or age, though the HC group’s education level was, on average, higher than that the other two groups [HC > cMDD = rMDD, F(2,196) = 12.023, p < 0.001]. Mean HAMD scores were greater in the cMDD group than in the rMDD group (t = 14.410, df = 119, p < 0.001). A one-way ANOVA revealed a main effect of group on BDI scores [F(2,196) = 310.668, p < 0.001] and post hoc analysis showed that each group’s BDI scores differed from those of the other two (cMDD > rMDD > HC, all post hoc p-values < 0.005).
ALFF Differences
With the one-way ANCOVA analysis, main effects of group on ALFF values were identified in the brain areas including the occipital and temporal cortices, as well as in the cerebellum (p = 0.001, uncorrected; see Figure 1A).
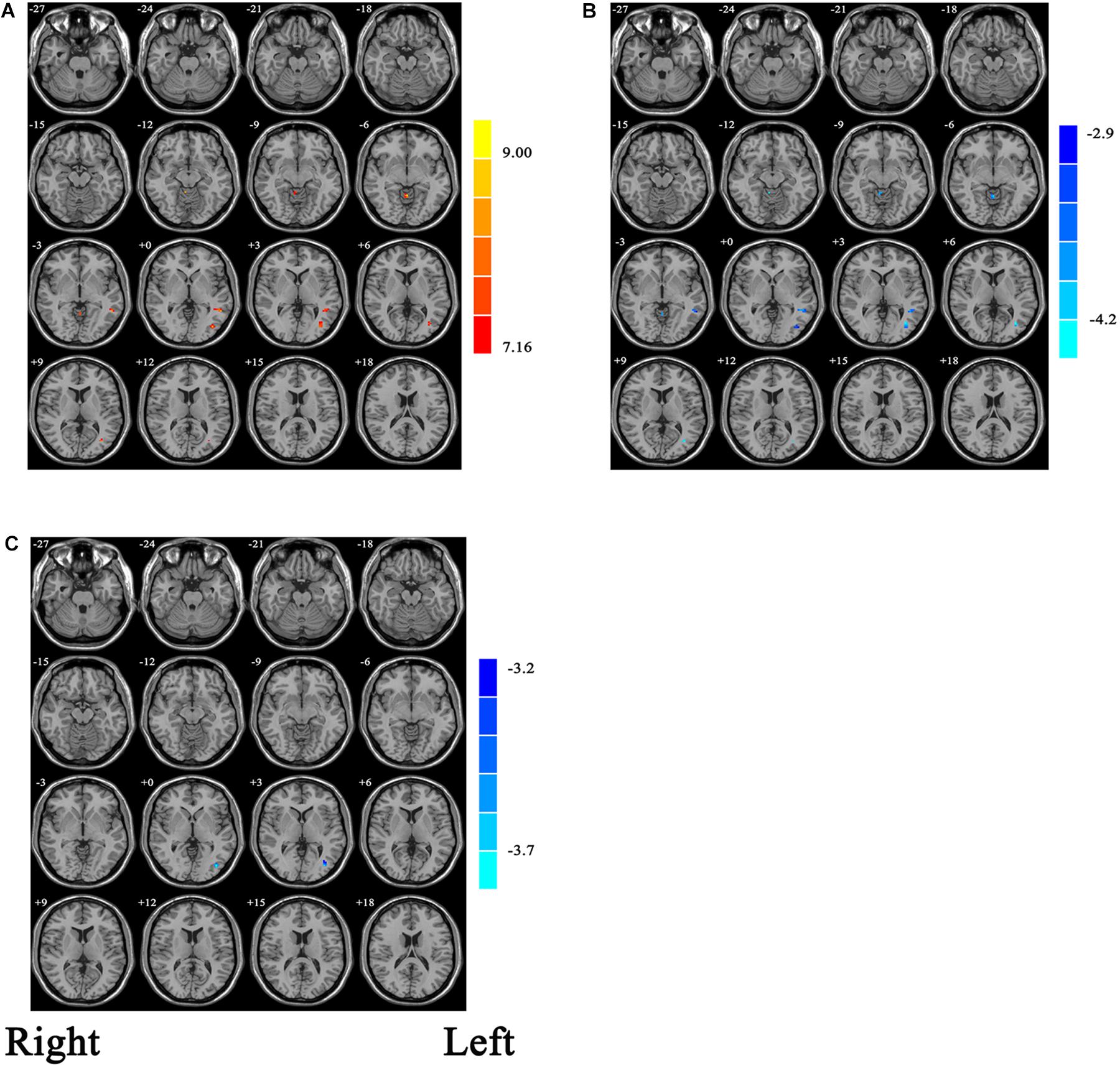
Figure 1. (A) Statistic maps showing ANOVA results of ALFF differences among current major depression disorder (cMDD), remitted major depression disorder (rMDD), and healthy control (HC) groups (p < 0.001, uncorrected). (B) Brain regions showing ALFF differences between cMDD and rMDD [p < 0.008, false discovery rate (FDR) corrected]. (C) Brain regions showing ALFF differences between cMDD and HC (p < 0.008, FDR corrected). ANOVA and post hoc t-tests were conducted using years of education as covariates of no interest. Two-sample t-test results are expressed within a mask showing significant group differences from the ANOVA. Red and blue denote ALFF increases and decreases, respectively; color bars indicate t-values.
Within the mask of these significant group differences, post hoc t-tests showed that, compared with the cMDD group, rMDD group had increased ALFF values in the left middle occipital gyrus, left middle temporal gyrus and right cerebellum anterior lobe (Table 2 and Figure 1B). Besides, compared with the HC group, the cMDD group had decreased ALFF values in the left middle occipital gyrus (Table 2 and Figure 1C). Furthermore, all p-values in the post hoc t-tests were corrected with false discovery rate (FDR) method (all p-values < 0.008; see Table 2).
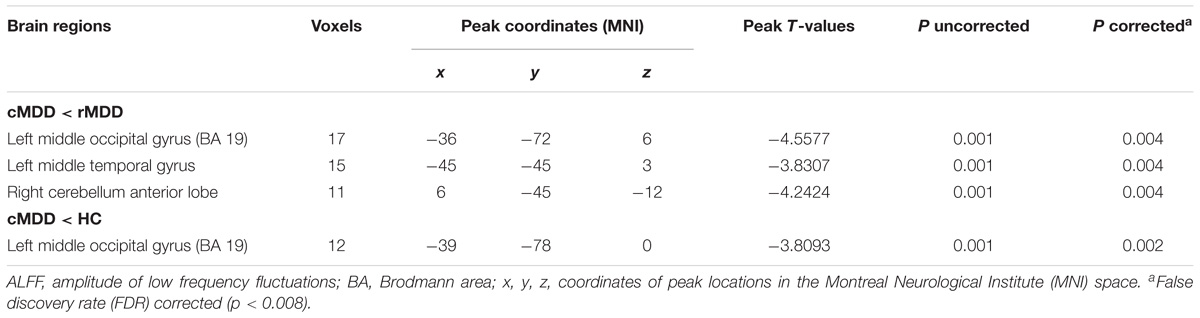
Table 2. Brain regions with significantly different ALFF values among the cMDD, rMDD, and HC groups.
Correlations Between ALFF Values and Clinical Variables
With respect to potential relationships between regional brain activity and clinical variables, mean ALFF values in the cMDD and rMDD groups, were separately obtained in the brain regions showing differences with the one-way ANCOVA analysis. The correlations between the mean ALFF values and BDI, HAMD scores were examined. Eventually, only the BDI scores in the rMDD group were correlated positively with ALFF values in the left middle occipital gyrus (r = 0.315, uncorrected p = 0.028; see Figure 2A), left middle temporal gyrus (r = 0.410, uncorrected p = 0.003; significant with Bonferroni correction [0.05/6]; see Figure 2B), right cerebellum anterior lobe (r = 0.299, uncorrected p = 0.037; see Figure 2C).
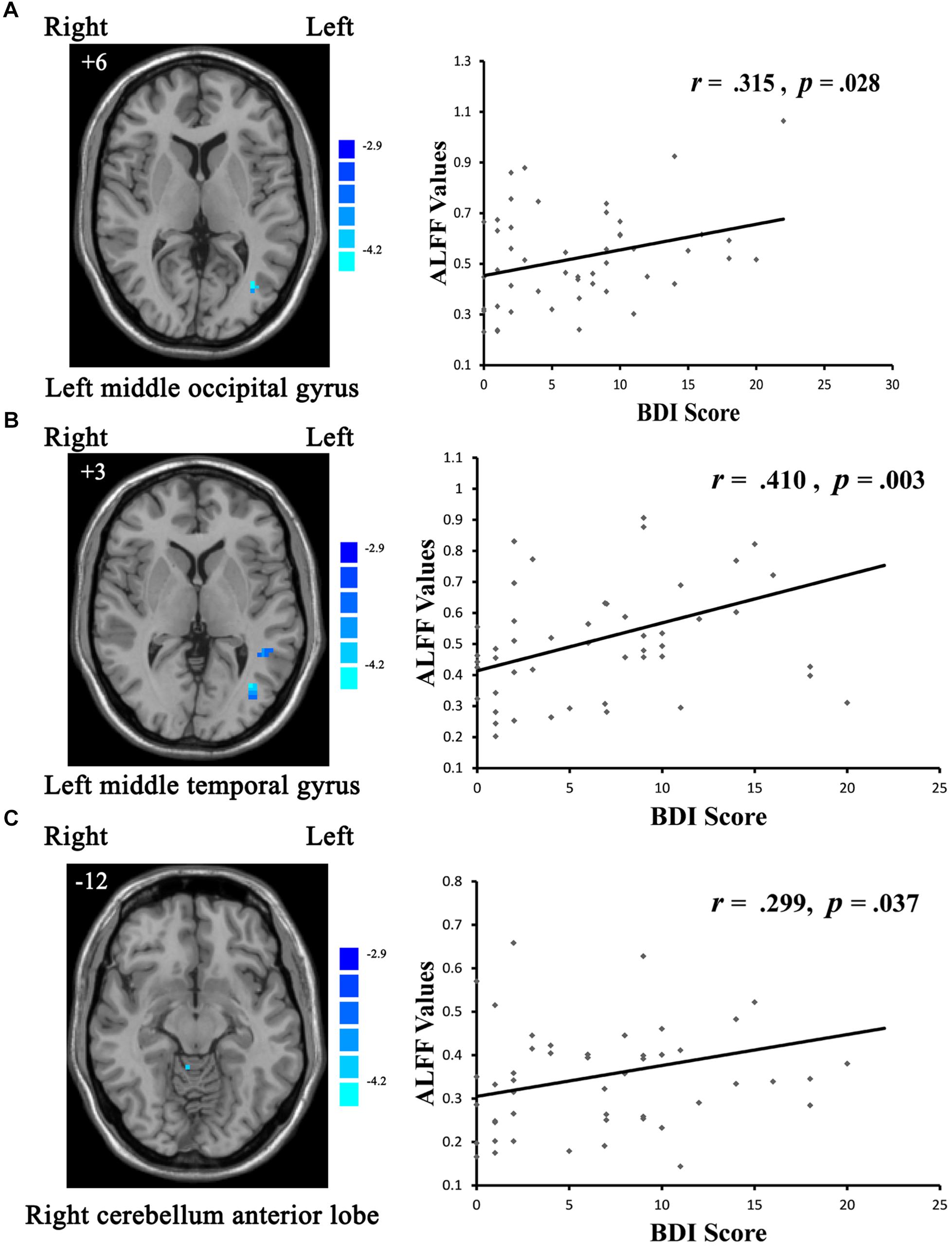
Figure 2. Scatter plots showing significant positive correlations between BDI scores and regional ALFF values in the (A) left middle occipital gyrus (uncorrected p = 0.028), (B) left middle temporal gyrus (uncorrected p = 0.003; significant with Bonferroni correction), (C) right cerebellum anterior lobe (uncorrected p = 0.037).
Discussion
The major finding of the current study was that the rMDD group showed increased hyperactivities in the left middle occipital gyrus, left middle temporal gyrus and right cerebellum anterior lobe when compared with the cMDD group by resting-state fMRI. Compared with the HC group, the cMDD group demonstrated decreased hypoactivity in the left middle occipital gyrus. The spontaneous neural activities in the left middle occipital gyrus, left middle temporal gyrus and right cerebellum anterior lobe were positively relevant with clinical symptom. These results suggested that brain activities in the left middle temporal gyrus, left middle occipital gyrus, and right cerebellum anterior lobe may serve as state-related biological characteristics of MDD.
The middle temporal gyrus, which is located between the superior temporal gyrus and inferior temporal gyrus, plays a vital role in the cognitive processing, such as language, memory and object vision processing (Onitsuka, 2004). Colombo and Gross (1994) reported that deficit in the middle temporal gyrus in monkeys would cause a poor performance in the cognitive task which requires visual object discrimination and recognition. A structural MRI study reported gray matter reduction in the middle temporal gyrus in treatment-resistant depression patients when compared with healthy subjects (Ma et al., 2012). Besides, the middle temporal gyrus is involved in the DMN (Zhou and Yu, 2010; Xue et al., 2016). Abnormal DMN functional alterations have been found closely bound up with rumination and autobiographical memory impairment in depression patients (Sumner et al., 2010; Paul et al., 2015).
The present study also found more hyperactivity in the middle temporal gyrus in the rMDD group compared with the cMDD group. This result and the relation between clinical symptom and brain activity in rMDD group may indicate the state-related functional compensation in the middle temporal gyrus. The functional compensation implies that the individuals suffered from damage of the central nervous system (CNS) would trigger the residual structures to achieve recovery, including behavioral, physical, or cognitive strategies (Tanaka, 2001). Furthermore, the temporal regions were deeply involved in social cognitive and affective processing (Wang et al., 2012). Therefore, we tentatively put forward that the state-related hyperactivity in the middle temporal gyrus may be involved in a compensatory mechanism, which is consistent with Goetz’s study that the compensatory effect has been found in the same brain region among the depression patients during a cognitive reappraisal task (Goetz et al., 2018).
Consistent with previous studies (Guo et al., 2013; Liang et al., 2013), the hyperactivity in the left middle occipital gyrus was found in MDD patients when compared with HC. The brain activity level in the left middle occipital gyrus was positively relevant with clinical symptom. The depressed patients with occipital brain activity abnormalities have shown a disproportionate attentional preference toward negative visual information (Guo et al., 2013). Depression-associated abnormalities of the middle occipital gyrus were related with abnormal neuropsychological activity, leading to a motor block and lowered attention (Yu et al., 2017). As described previously, this increased brain activity may be due to the state-related compensatory effect in the left middle occipital gyrus, which plays an active role in cognitive processing, including visual information processing and verbal episodic memory (Li et al., 2016).
The cerebellum, a structure used to be most appreciated for its important role in motor coordination and behavior (Stein, 1986), was reported taking part in emotional and cognitive processing in recent depression studies (Schmahmann, 2000; Lekeu et al., 2002; Lin et al., 2012). Our study found increased activities in the anterior lobe of cerebellum in the rMDD group than in the cMDD group. Besides, the activity level in this area was correlated with depressive symptom in the rMDD group, which may imply that the state-related hyperactivities in the anterior lobe of cerebellum found in the rMDD patients are involved in a compensatory mechanism. The relation between cerebellum brain activity and depressive symptom has been reported by fMRI and sMRI findings (Zeng et al., 2012; Xu et al., 2017). This association may be illustrated by the cerebellar connections with limbic regions, brainstem, temporal lobe, prefrontal lobe, and cingulate gyrus, areas shown to have profound influence on cognitive processing and emotional regulation (Haines et al., 1984; Middleton and Strick, 2001; Turner et al., 2007). A growing number of studies have paid attention to the role of cerebellum in depression (Lin et al., 2012; Phillips et al., 2015; Ming Z.M. et al., 2017), and our research implied that the anterior cerebellum may be related with the depression remitted process.
Limitations
Our study has some limitations that need to be addressed in further studies. Firstly, we used a cross-sectional study design. Longitudinal studies are required to clarify how neural brain activities evolve from depression onset to remission, and from remission to recurrence. Second, to exclude potential confounders, we excluded patients with comorbidities. However, more than three-quarters of MDD patients have comorbidities (Kessler et al., 2003). Thus, it is not known whether the state-related features found in this study would be detectable in MDD patients with comorbidities. Third, in our study, the ANCOVA anlysis result of ALFF values was uncorrected and voxels cluster size was small for a clusterwise correction. Therefore, our findings, as an exploring research, may only imply a trend of the abnormal activity brain area in cMDD, rMDD and HC groups. Longitudinal studies across different clinical states would be required in the future exploration.
Conclusion
To our knowledge, this is the first study to explore state-related alterations of spontaneous neural activity in medication-naïve current and remitted depression. Consistent with the research hypothesis, state-related abnormal spontaneous neural activities were observed in the DMN and cerebellum, including the middle temporal gyrus, cerebellum anterior lobe. Furthermore, the state-related compensatory effect was found in the middle occipital gyrus, middle temporal gyrus and cerebellum anterior lobe. Although these findings remain to be confirmed, our study provides a fresh perspective for elucidating the neurobiology of MDD development, maintenance and recovery. The state-related characteristic of MDD suggested by our study may be useful for improving clinical diagnosis and prognostic evaluation of MDD, planning of targeted interventions, and monitoring of therapeutic efficacy.
Author Contributions
SY supervised the study. CC performed the analysis and wrote paper. DD, YJ, and QM contributed to the analysis. XZ, XS, GX, and YG collected the data. All co-authors revised and approved the version to be published.
Funding
This research was supported by the National Natural Science Foundation of China (Grant Number 81471384).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
References
Ayloo, S., Thompson, K., Choudhury, N., and Sheriffdeen, R. (2015). Correlation between the beck depression inventory and bariatric surgical procedures. Surg. Obesity Relat. Dis. 11, 637–642. doi: 10.1016/j.soard.2014.11.005
Beck, A. T., Steer, R. A., and Brown, G. K. (1996). Manual for the Beck Depression Inventory-II. Washington, DC: American University, 21.
Biswal, B. B. (2012). Resting state fMRI: a personal history. Neuroimage 62, 938–944. doi: 10.1016/j.neuroimage.2012.01.090
Caetano, S. C., Hatch, J. P., Brambilla, P., Sassi, R. B., Nicoletti, M., Mallinger, A. G., et al. (2004). Anatomical MRI study of hippocampus and amygdala in patients with current and remitted major depression. Psychiatry Res. 132, 141–147. doi: 10.1016/j.pscychresns.2004.08.002
Colombo, M., and Gross, C. G. (1994). Responses of inferior temporal cortex and hippocampal neurons during delayed matching-to-sample in monkeys (Macaca fascicularis). Behav. Neurosci. 108, 443–455. doi: 10.1037/0735-7044.108.3.443
Dong, D., Ming, Q., Zhong, X., Pu, W., Zhang, X., Jiang, Y., et al. (2018). State-independent alterations of intrinsic brain network in current and remitted depression. Prog. Neuro Psychopharmacol. Biol. Psychiatry 89, 475–480. doi: 10.1016/j.pnpbp.2018.08.031
Fan, J., Zhong, M., Gan, J., Liu, W., Niu, C., Liao, H., et al. (2017). Spontaneous neural activity in the right superior temporal gyrus and left middle temporal gyrus is associated with insight level in obsessive-compulsive disorder. J. Affect. Disord. 207, 203–211. doi: 10.1016/j.jad.2016.08.027
First, M., Spitzer, R., Gibbon, M., and Williams, J. (2002). Structured Clinical Interview for DSM-IV-TR Axis I Disorders–Patient Edtion (SCID-I/P, 11/2002 revision). New York, NY: New York State Psychiatric Institute.
Goetz, D. S. E., Foland-Ross, L. C., and Gotlib, I. H. (2018). Neural correlates of top-down regulation and generation of negative affect in major depressive disorder. Psychiatry Res. Neuroimag. 276, 1–8. doi: 10.1016/j.pscychresns.2018.04.001
Goulden, N., Mckie, S., Thomas, E. J., Downey, D., Juhasz, G., Williams, S. R., et al. (2012). Reversed frontotemporal connectivity during emotional face processing in remitted depression. Biol. Psychiatry 72, 604–611. doi: 10.1016/j.biopsych.2012.04.031
Guo, W. B., Liu, F., Xun, G. L., Hu, M. R., Guo, X. F., Xiao, C. Q., et al. (2013). Reversal alterations of amplitude of low-frequency fluctuations in early and late onset, first-episode, drug-naive depression. Prog. Neuro Psychopharmacol. Biol. Psychiatry 40, 153–159. doi: 10.1016/j.pnpbp.2012.08.014
Haines, D. E., Dietrichs, E., and Sowa, T. E. (1984). Hypothalamo-cerebellar and cerebello-hypothalamic pathways: a review and hypothesis concerning cerebellar circuits which may influence autonomic centers and affective behavior (part 1 of 2). Brain Behav. Evol. 24, 198–209. doi: 10.1159/000121317
Hamilton, M. (1960). A rating scale for depression. J. Neurol. Neurosurg. Psychiatry 23, 56–62. doi: 10.1136/jnnp.23.1.56
Hardeveld, F., Spijker, J., Graaf, R. D., Nolen, W. A., and Beekman, A. T. F. (2010). Prevalence and predictors of recurrence of major depressive disorder in the adult population. Acta Psychiatr. Scand. 122, 184–191. doi: 10.1111/j.1600-0447.2009.01519.x
Henson, R. N. A., Shallice, T., Gorno-Tempini, M. L., and Dolan, R. J. (2002). Face repetition effects in implicit and explicit memory tests as measured by fMRI. Cereb. Cortex 12, 178–186. doi: 10.1093/cercor/12.2.178
Ji, J., Kleinman, A., and Becker, A. E. (2001). Suicide in contemporary China: a review of China’s distinctive suicide demographics in their sociocultural context. Harvard Rev. Psychiatry 9, 1–12. doi: 10.1080/10673220127875
Jing, B., Liu, C. H., Ma, X., Yan, H. G., Zhuo, Z. Z., Zhang, Y., et al. (2013). Difference in amplitude of low-frequency fluctuation between currently depressed and remitted females with major depressive disorder. Brain Res. 1540, 74–83. doi: 10.1016/j.brainres.2013.09.039
Kessler, R. C., Berglund, P., Demler, O., Jin, R., Koretz, D., Merikangas, K. R., et al. (2003). The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication. J. Am. Med. Assoc. 289, 3095–3105. doi: 10.1001/jama.289.23.3095
Lee, M. H., Smyser, C. D., and Shimony, J. S. (2013). Resting state fMRI: a review of methods and clinical applications. Am. J. Neuroradiol. 34, 1866–1872. doi: 10.3174/ajnr.A3263
Lekeu, F., Marczewski, P., Van Der Linden, M., Collette, F., Degueldre, C., Del Fiore, G., et al. (2002). Effects of incidental and intentional feature binding on recognition: a behavioral and PET activation study. Neuropsychologia 40, 131–144. doi: 10.1016/S0028-3932(01)00088-4
Li, G., Ma, X., Bian, H., Sun, X., Zhai, N., Yao, M., et al. (2016). A pilot fMRI study of the effect of stressful factors on the onset of depression in female patients. Brain Imag. Behav. 10, 195–202. doi: 10.1007/s11682-015-9382-8
Liang, M. J., Zhou, Q., Yang, K. R., Yang, X. L., Fang, J., Chen, W. L., et al. (2013). Identify changes of brain regional homogeneity in bipolar disorder and unipolar depression using resting-state FMRI. PLoS One 8:e79999. doi: 10.1371/journal.pone.0079999
Lin, W. C., Chou, K. H., Chen, H. L., Huang, C. C., Lu, C. H., Li, S. H., et al. (2012). Structural deficits in the emotion circuit and cerebellum are associated with depression, anxiety and cognitive dysfunction in methadone maintenance patients: a voxel-based morphometric study. Psychiatry Res. 201, 89–97. doi: 10.1016/j.pscychresns.2011.05.009
Liu, C. H., Jing, B., Ma, X., Xu, P. F., Zhang, Y., Li, F., et al. (2014). Voxel-based morphometry study of the insular cortex in female patients with current and remitted depression. Neuroscience 262, 190–199. doi: 10.1016/j.neuroscience.2013.12.058
Logothetis, N. K., Pauls, J., Augath, M., Trinath, T., and Oeltermann, A. (2001). Neurophysiological investigation of the basis of the fMRI signal. Nature 412, 150–157. doi: 10.1038/35084005
Lorenzetti, V., Allen, N. B., Whittle, S., and Yucel, M. (2010). Amygdala volumes in a sample of current depressed and remitted depressed patients and healthy controls. J. Affect. Disord. 120, 112–119. doi: 10.1016/j.jad.2009.04.021
Ma, C., Ding, J., Li, J., Guo, W., Long, Z., Liu, F., et al. (2012). Resting-state functional connectivity bias of middle temporal gyrus and caudate with altered gray matter volume in major depression. PLoS One 7:e45263. doi: 10.1371/journal.pone.0045263
Mayberg, H. S. (1997). Limbic-cortical dysregulation: a proposed model of depression. J. Neuropsychiatry Clin. Neurosci. 9, 471–481. doi: 10.1176/jnp.9.3.471
Mayberg, H. S. (2003). Modulating dysfunctional limbic-cortical circuits in depression: towards development of brain-based algorithms for diagnosis and optimised treatment. Br. Med. Bull. 65, 193–207. doi: 10.1093/bmb/65.1.193
Middleton, F. A., and Strick, P. L. (2001). Cerebellar projections to the prefrontal cortex of the primate. J. Neurosci. 21, 700–712. doi: 10.1523/JNEUROSCI.21-02-00700.2001
Ming, Q., Zhong, X., Zhang, X., Pu, W., Dong, D., Jiang, Y., et al. (2017). State-independent and dependent neural responses to psychosocial stress in current and remitted depression. Am. J. Psychiatry 174, 971–979. doi: 10.1176/appi.ajp.2017.16080974
Ming, Z. M., Hu, X., Lu, L. M., Liangqing Zhang, M. M., Chen, L., Gong, Q., et al. (2017). Intrinsic cerebral activity at resting state in adults with major depressive disorder: a meta-analysis. Prog. Neuropsychopharmacol. Biol. Psychiatry 75, 157–164. doi: 10.1016/j.pnpbp.2017.02.001
Onitsuka, T. (2004). Middle and inferior temporal gyrus gray matter volume abnormalities in chronic schizophrenia: an MRI study. Am. J. Psychiatry 161, 2103–2110. doi: 10.1176/appi.ajp.161.9.1603
Paul, H. J., Madison, F., Phoebe, F., and Gotlib, I. H. (2015). Depressive rumination, the default-mode network, and the dark matter of clinical neuroscience. Biol. Psychiatry 78, 224–230. doi: 10.1016/j.biopsych.2015.02.020
Phillips, J. R., Hewedi, D. H., Eissa, A. M., and Moustafa, A. A. (2015). The cerebellum and psychiatric disorders. Front. Public Health 3:66. doi: 10.3389/fpubh.2015.00066
Satterthwaite, T. D., Elliott, M. A., Gerraty, R. T., Ruparel, K., Loughead, J., Calkins, M. E., et al. (2013). An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting-state functional connectivity data. Neuroimage 64, 240–256. doi: 10.1016/j.neuroimage.2012.08.052
Schaefer, H. S., Putnam, K. M., Benca, R. M., and Davidson, R. J. (2006). Event-related functional magnetic resonance imaging measures of neural activity to positive social stimuli in pre- and post-treatment depression. Biol. Psychiatry 60, 974–986. doi: 10.1016/j.biopsych.2006.03.024
Schmahmann, J. D. (2000). The role of the cerebellum in affect and psychosis. J. Neurolinguist. 13, 189–214. doi: 10.1016/S0911-6044(00)00011-7
Song, X. W., Dong, Z. Y., Long, X. Y., Li, S. F., Zuo, X. N., Zhu, C. Z., et al. (2011). REST: a toolkit for resting-state functional magnetic resonance imagingdata processing. PLoS One 6:e25031. doi: 10.1371/journal.pone.0025031
Stein, J. F. (1986). Role of the cerebellum in the visual guidance of movement. Nature 323, 217–221. doi: 10.1038/323217a0
Su, L., Li, T., Deng, W., Jiang, L., Wu, Q., Tang, H., et al. (2010). Short-term effects of antipsychotic treatment on cerebral function in drug-naive first-episode schizophrenia revealed by “resting state” functional magnetic resonance imaging. Arch. Gen. Psychiatry 67, 783–792. doi: 10.1001/archgenpsychiatry.2010.84
Sumner, J. A., Griffith, J. W., and Susan, M. (2010). Overgeneral autobiographical memory as a predictor of the course of depression: a meta-analysis. Behav. Res. Ther. 48, 614–625. doi: 10.1016/j.brat.2010.03.013
Takami, H., Okamoto, Y., Yamashita, H., Okada, G., and Yamawaki, S. (2007). Attenuated anterior cingulate activation during a verbal fluency task in elderly patients with a history of multiple-episode depression. Am. J. Geriatr. Psychiatry 15, 594–603. doi: 10.1097/01.JGP.0b013e31802ea919
Tanaka, K. (2001). Temporal lobe. Int. Encycloped. Soc. Behav. Sci. 28, 15595–15599. doi: 10.1016/B0-08-043076-7/03469-0
Tomasi, D., Wang, G. J., and Volkow, N. D. (2013). Energetic cost of brain functional connectivity. Proc. Natl. Acad. Sci. U.S.A. 110, 13642–13647. doi: 10.1073/pnas.1303346110
Turner, B. M., Paradiso, S., Marvel, C. L., Pierson, R., Boles Ponto, L. L., Hichwa, R. D., et al. (2007). The cerebellum and emotional experience. Neuropsychologia 45, 1331–1341. doi: 10.1016/j.neuropsychologia.2006.09.023
van Eijndhoven, P., Van Wingen, G., Katzenbauer, M., Groen, W., Tepest, R., Fernandez, G., et al. (2013). Paralimbic cortical thickness in first-episode depression: evidence for trait-related differences in mood regulation. Am. J. Psychiatry 170, 1477–1486. doi: 10.1176/appi.ajp.2013.12121504
Wang, L., Dai, W., Su, Y., Wang, G., Tan, Y., Jin, Z., et al. (2012). Amplitude of low-frequency oscillations in first-episode, treatment-naive patients with major depressive disorder: a resting-state functional mri study. PLoS One 7:e48658. doi: 10.1371/journal.pone.0048658
Wang, L., Krishnan, K. R., Steffens, D. C., Potter, G. G., Dolcos, F., and Mccarthy, G. (2008). Depressive state- and disease-related alterations in neural responses to affective and executive challenges in geriatric depression. Am. J. Psychiatry 165, 863–871. doi: 10.1176/appi.ajp.2008.07101590
Waugh, C. E., Hamilton, J. P., Chen, M. C., Joormann, J., and Gotlib, I. H. (2012). Neural temporal dynamics of stress in comorbid major depressive disorder and social anxiety disorder. Biol. Mood Anxiety Disord. 2, 1–15. doi: 10.1186/2045-5380-2-11
Xu, L. Y., Xu, F. C., Liu, C., Ji, Y. F., Wu, J. M., Wang, Y., et al. (2017). Relationship between cerebellar structure and emotional memory in depression. Brain Behav. 7:e00738. doi: 10.1002/brb3.738
Xue, Z., Pu, W., and Yao, S. (2016). Functional alterations of fronto-limbic circuit and default mode network systems in first-episode, drug-naïve patients with major depressive disorder: a meta-analysis of resting-state fMRI data. J. Affect. Disord. 206, 280–286. doi: 10.1016/j.jad.2016.09.005
Yan, C. G., and Zang, Y. F. (2010). DPARSF: a MATLAB toolbox for “pipeline” data analysis of resting-state fMRI. Front. Syst. Neurosci. 4:13. doi: 10.3389/fnsys.2010.00013
Yu, H. L., Liu, W. B., Wang, T., Huang, P. Y., Jie, L. Y., Sun, J. Z., et al. (2017). Difference in resting-state fractional amplitude of low-frequency fluctuation between bipolar depression and unipolar depression patients. Eur. Rev. Med. Pharmacol. Sci. 21, 1541–1550.
Zang, Y. F., He, Y., Zhu, C. Z., Cao, Q. J., Sui, M. Q., Liang, M., et al. (2007). Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev. 29, 83–91. doi: 10.1016/j.braindev.2006.07.002
Zeng, L. L., Shen, H., Liu, L., Wang, L., Li, B., Fang, P., et al. (2012). Identifying major depression using whole-brain functional connectivity: a multivariate pattern analysis. Brain 135, 1498–1507. doi: 10.1093/brain/aws059
Zhang, X., Zhu, X., Wang, X., Zhu, X., Zhong, M., Yi, J., et al. (2014). First-episode medication-naive major depressive disorder is associated with altered resting brain function in the affective network. PLoS One 9:e85241. doi: 10.1371/journal.pone.0085241
Keywords: major depressive disorder, remission, trait-related, state-related, resting-state fMRI, amplitude of lowfrequency fluctuation
Citation: Cheng C, Dong D, Jiang Y, Ming Q, Zhong X, Sun X, Xiong G, Gao Y and Yao S (2019) State-Related Alterations of Spontaneous Neural Activity in Current and Remitted Depression Revealed by Resting-State fMRI. Front. Psychol. 10:245. doi: 10.3389/fpsyg.2019.00245
Received: 06 September 2018; Accepted: 24 January 2019;
Published: 11 February 2019.
Edited by:
James Alan Bourgeois, Baylor Scott & White Health, United StatesCopyright © 2019 Cheng, Dong, Jiang, Ming, Zhong, Sun, Xiong, Gao and Yao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shuqiao Yao, shuqiaoyao@csu.edu.cn
 Chang Cheng1,2
Chang Cheng1,2 Yali Jiang
Yali Jiang Qingsen Ming
Qingsen Ming Shuqiao Yao
Shuqiao Yao