fNIRS Studies on Hemispheric Asymmetry in Atypical Neural Function in Developmental Disorders
- Department of Neurobiology and Behavior, Graduate School of Biomedical Sciences, Nagasaki University, Nagasaki, Japan
Functional lateralization is highly replicable trait of human neural system. Many previous studies have indicated the possibility that people with attention-deficits/hyperactivity-disorder (ADHD) and autism spectrum disorder (ASD) show hemispheric asymmetry in atypical neural function. However, despite the abundance of relevant studies, there is still ongoing controversy over this issue. In the present mini-review, we provide an overview of the hemispheric asymmetry in atypical neural function observed in fNIRS studies on people with these conditions. Atypical neural function is defined as group-difference in the task-related concentration change of oxygenated hemoglobin. The existing fNIRS studies give support to the right-lateralized atypicalty in children with ADHD. At the same time, we did not find clear leftward-lateralization in atypical activation in people with ASD. On the basis of these, we discuss the current states and limitation of the existing studies.
Lateralization in Atypical Neural Function in Developmental Disorders
Functional near-infrared spectroscopy (fNIRS) was introduced into the scientific community as a neuroimaging tool ~20 years ago (Hoshi and Tamura, 1993; Kato et al., 1993). Despite having relatively poor spatial and temporal resolution compared to fMRI and EEG/MEG respectively, fNIRS is associated with certain advantages over other non-invasive techniques for measuring neural function. For instance, fNIRS poses a low physical and psychological burden on participants. Additionally, fNIRS is less vulnerable to artifacts generated by bodily motion. These features are particularly advantageous for measuring neural function in individuals with pathological conditions (Doi et al., 2013; Koike et al., 2013; Adorni et al., 2016).
Besides them, fNIRS has some unique characteristics compared to the other non-invasive measurements of neural function. First, in contrast to EEG, which measures the electrical activity (primary signal) pooled across wide neural regions, fNIRS measures hemodynamic response (secondary signal) with relatively high spatial resolusion. Second, the concentrations of oxygenated-/deoxygenated hemoglobin (oxyHb/deoxyHb) measured by fNIRS reflect aspects of hemodynamic response that are different from the indicators used in other neuroimaging techniques (Minagawa-Kawai et al., 2009a). Relative increase of oxyHb concomitant with slight decrease of deoxyHb is supposed to reflect the influx of oxyHb to the blood vessels adjascent to activated cortical region to meet the demands of energy consumption by neurons in the region (Minagawa-Kawai et al., 2009a; Doi et al., 2013). In contrast to this, the BOLD signal measured in fMRI technique is considered to mainly reflect the decrease of deoxyHb (Song et al., 2006), although the physiological basis of BOLD signal remains elusive at this point. Therefore, incorporating findings from fNIRS studies might lead to a more comprehensive understanding of typical and atypical patterns of neural function.
Functional lateralization has been repeatedly documented in the human neural system; a number of studies have generally shown leftward-lateralization of linguistic function (Crow, 2000) and right-lateralization of attentional function and visuo-spatial cognition (Toga and Thompson, 2003; Hervé et al., 2013). There is a long history of studies investigating lateralization in atypical neural function in developmental disorders (McCann, 1982; see, Klimkeit and Bradshaw, 2006, for a brief review). However, despite the abundance of relevant studies, there is still controversy over whether people with developmental disorders exhibit lateralization in atypical neural function.
Since its introduction, the number of fNIRS studies focused on people with developmental disorders has been steadily increasing (for a review, Ehlis et al., 2014). Because the majority of these studies have used bilaterally-placed multichannel emitter-detector probe sets, the resulting datasets offer an invaluable opportunity to examine lateralization in atypical neural function in individuals with developmental disorders.
Aim
Here we provide a qualitative overview of the existing fNIRS studies of individuals with developmental disorders, with a specific focus on lateralization in atypical neural function. Although several reviews of fNIRS research have been published (Doi et al., 2013; Koike et al., 2013; Ehlis et al., 2014; Balconi et al., 2015; Adorni et al., 2016), to the best of our knowledge, this is the first to focus on this aspect. The conditions discussed here include attention-deficit/hyperactivity disorder (ADHD) and autism spectrum disorder (ASD). Previous studies have indicated the possibility that individuals with these conditions (McCann, 1982; Klimkeit and Bradshaw, 2006) show lateralization in atypical neural function, but these findings are not often consolidated into theoretical overviews. Therefore, our primary goal here is to establish a scaffolding for the organization and consolidation of findings obtained using fNIRS regarding the lateralization in atypical neural function in people with ADHD and ASD.
As per convention, we treat task-related increases in oxyHb as the primary indicator of neural function (Minagawa-Kawai et al., 2009a; Doi et al., 2013). The atypicality of neural function observed so far comes mainly in three forms. First, some studies have quantitatively compared oxyHb changes between patient and control groups. As a result, many studies found statistically significant between-group differences in the level of task-related oxyHb change in either one or both hemispheres. In the second type of atypicality, hemispheric asymmetry is observed in either the patient or control group, but not in both. More specifically, in some such cases the patient group does not show the lateralized oxyHb changes observed in the control group (lack of lateralization), while in others patients show lateralization not normally observed in matched-controls. Third, several studies have revealed a lack of significant task-related changes in oxyHb from the preceding baseline period in the patient group in either one of the hemispheres when matched-controls showed significant task-related changes.
Of the three types of atypicality described above, we focus mainly on the first, as only this type is ascertained by the direct comparison of patient and matched-control groups. For descriptive brevity, we refer to such reduced/enhanced levels of task-related oxyHb increase in patients compared with matched controls as “hypo-/hyper-activation.” In the following, the term “lateralization in atypical neural function/activation” refers mainly to hypo-/hyper-activation being observed only in one hemisphere.
Lateralization in Atypical Neural Function in ADHD
ADHD is a developmental disorder with inattention, impulsivity, and hyperactivity as core symptoms. Children with ADHD often have poor social skills and learning disabilities. Approximately 10% of school-aged children and 5% of adults are estimated to suffer from ADHD (Pietrzak et al., 2006; Safren et al., 2010; Thomas et al., 2015).
It has long been postulated that the symptoms of ADHD are associated with right-hemisphere abnormalities (Stefanatos and Wasserstein, 2001). This is largely because functions such as attentional control, visuo-spatial processing, and socio-emotional processing, for which ADHD children show relatively poor performance, are generally right-lateralized in typically developing people (Toga and Thompson, 2003; Hervé et al., 2013). This notion has gained support from studies utilizing behavioral experiments and neuroimaging techniques (for review, Stefanatos and Wasserstein, 2001; Valera et al., 2007). However, several recent studies have shown a more nuanced pattern of atypical lateralization (Silk et al., 2016) or have shown atypical interhemispheric integration (Hale et al., 2009) in ADHD.
The number of fNIRS studies of individuals with ADHD is relatively small, but these generally support right-lateralized atypicality in wide cortical regions in ADHD. To further verify this observation, we surveyed relevant peer-reviewed studies using the Scopus database. We mainly included studies that compared task-related oxyHb changes in bilaterally-placed channels, between people with ADHD and matched controls. Conference proceedings and review papers were excluded. This resulted in a total of 24 eligible studies. The details of these studies are summarized in Table 1. The distribution of observed group-differences are described in Figure 1.
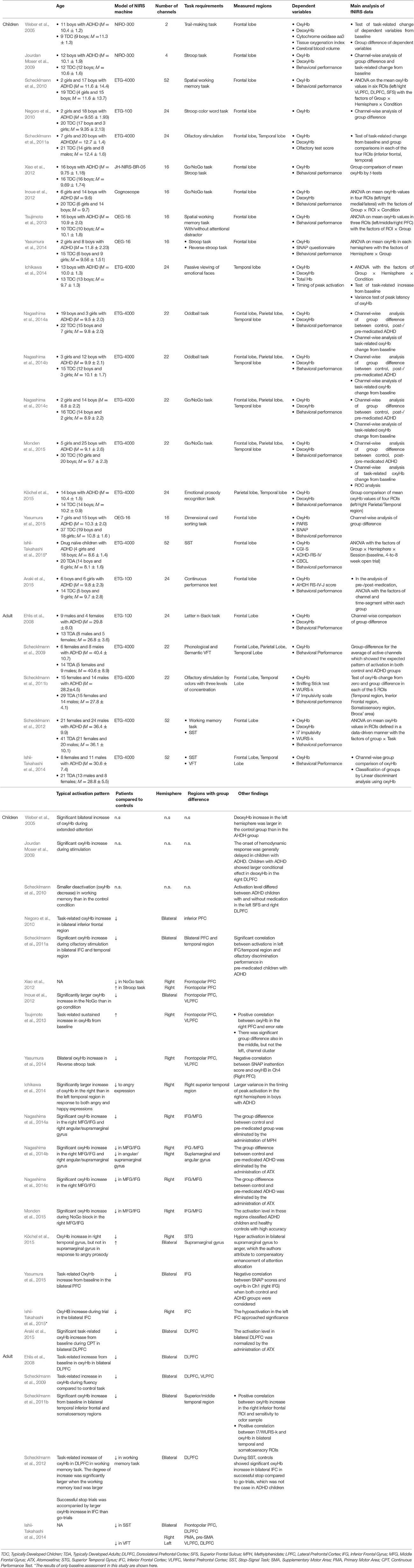
Table 1. The details of the main fNIRS studies on people with ADHD explained in the present mini-review: only the results of group comparison with matched-controls are shown.
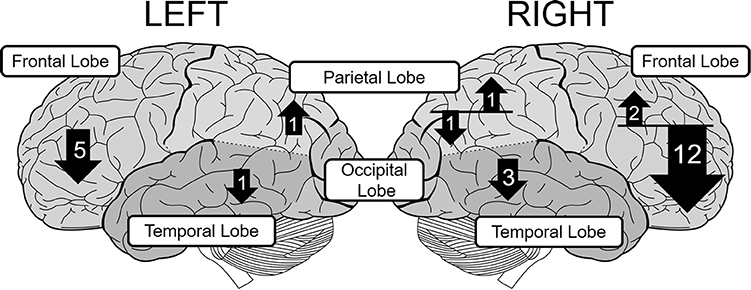
Figure 1. The distribution of group-differences in each lobe in children with ADHD. The upward and downward arrows represent hyper- and hypoactivation, respectively. The number in each arrow represents the number of papers that found statistically significant group difference. The size of each arrow is not strictly proportional to the number of papers.
Most of the studies of children with ADHD show atypical patterns of oxyHb more prominently in the right hemisphere during a variety of tasks such as the reverse-Stroop task (Yasumura et al., 2014), executive attention control task (Tsujimoto et al., 2013), verbal fluency task (VFT; Schecklmann et al., 2009), Go/NoGo task (Monden et al., 2012, 2015; Xiao et al., 2012; Nagashima et al., 2014a), oddball task (Nagashima et al., 2014b,c), passive viewing of facial expression (Ichikawa et al., 2014), and emotional prosody recognition (Köchel et al., 2015). These studies have revealed hypoactivation in the right frontal lobe including the prefrontal cortex (PFC; Xiao et al., 2012; Yasumura et al., 2014), middle frontal gyrus (MFG), and inferior frontal gyrus (IFG) (Monden et al., 2012, 2015; Nagashima et al., 2014a,b,c), presumably because NIRS probes can easily be applied to the frontal region (see Table 1). These studies also found hypoactivation in the temporal (Ichikawa et al., 2014; Köchel et al., 2015) and parietal cortices (Nagashima et al., 2014b) as well.
Interestingly, a few studies found atypicality in the pattern of deoxyHb alteration in children with ADHD (Weber et al., 2005; Jourdan Moser et al., 2009). For example, Weber et al. (2005) reported larger deoxyHb increase in the left superior/middle frontal cortex in controls than children with ADHD, without group difference in oxyHb alteration. Low level of deoxyHb increase may reflect inefficient oxygen consumption due to redcued cortical activation. Thus, incorporating the findings on deoxyHb may give us more comprehensive picture about the hemispheric asymmetry in atypical neural function in people with ADHD, although these findings are sporadic at this point.
While the majority of studies that recruited children with ADHD report right-lateralized frontal hypoactivation (Monden et al., 2012, 2015; Xiao et al., 2012; Nagashima et al., 2014a,b,c; Yasumura et al., 2014), bilateral frontal hypoactivation seems more prevalent among adults with ADHD (Ehlis et al., 2008; Schecklmann et al., 2011b). The ADHD symptoms in children are reported to become less severe as they get older, which partly explains the lower prevalence rate of ADHD in adults than pediatric population (Pietrzak et al., 2006; Safren et al., 2010; Thomas et al., 2015). Considering this, the more wide-spread PFC hypoactivation in adults with ADHD raises the possibility that these patients constitute sub-group with severe form of ADHD, whose symptoms persist despite development. However, as the number of fNIRS studies of adult ADHD patients is disproportionately small, this observation requires further empirical validation.
Lateralization in Atypical Neural Function in ASD
ASD is an umbrella term collectively referring to heterogenous groups of individuals who share the following core symptoms: Deficits in socio-communicative ability, fixed or restricted behaviors, and repetitive patterns of behavior (APA, 2013). ASD has several sub-groups that differ in symptomatic profiles and cognitive-emotional ability such as intellectual and linguistic prowess (Lenroot and Yeung, 2013).
Since the early days of autism research, investigators have posited that the symptoms of ASD are associated with atypical left-hemisphere function, largely based on the observation that children with Kanner's autism have impaired linguistic ability (McCann, 1982). Later studies reported reduced leftward lateralization in people with ASD with (De Fossé et al., 2004) or without language delay (Floris et al., 2016). That is, people with ASD show weaker level of leftward lateralization in linguistic function than typically developed people. Furthermore, recent resting-state fMRI studies have shown weaker interhemispheric communication (Anderson et al., 2011) and an increased degree of rightward lateralization in the resting-state activity of non-language brain regions recruited during visual/tactile perception, motor-planning, and executive functioning (Cardinale et al., 2013).
To review fNIRS studies of people with ASD, we searched for relevant papers using the Scopus database. Similar criteria to that described in lateralization in atypical neural function in ADHD were adopted in selecting eligible studies. The details of these are summarized in Table 2. Most of these studies have refuted the notion of a leftward-lateralization in atypical function in ASD by showing bilateral hypoactivation in the frontal cortex including IFG/motor-related cortices (Kajiume et al., 2013), and dorsolateral PFC (DLPFC)/frontopolar PFC (Kawakubo et al., 2009; Iwanami et al., 2011; Iwanaga et al., 2013; Ishii-Takahashi et al., 2014) using tasks such as the VFT (Kuwabara et al., 2006; Kawakubo et al., 2009; Iwanami et al., 2011), mental-state reading task (Iwanaga et al., 2013), stop-signal task (SST; Ishii-Takahashi et al., 2014), and imitation task (Kajiume et al., 2013). In contrast to ADHD, no clear difference was observed between adult and pediatric population with ASD in the lateralization pattern in atypical neural function. A few of the studies showing bilateral hypoactivation report hypoactivation in wider cortical regions in the left than in the right hemisphere (Ishii-Takahashi et al., 2014). For example, Ishii-Takahashi et al. (2014) found hypoactivation during SST in the left ventrolateral PFC (VLPFC) and motor-related areas, in addition to the bilateral DLPFC/ frontopolar PFC.
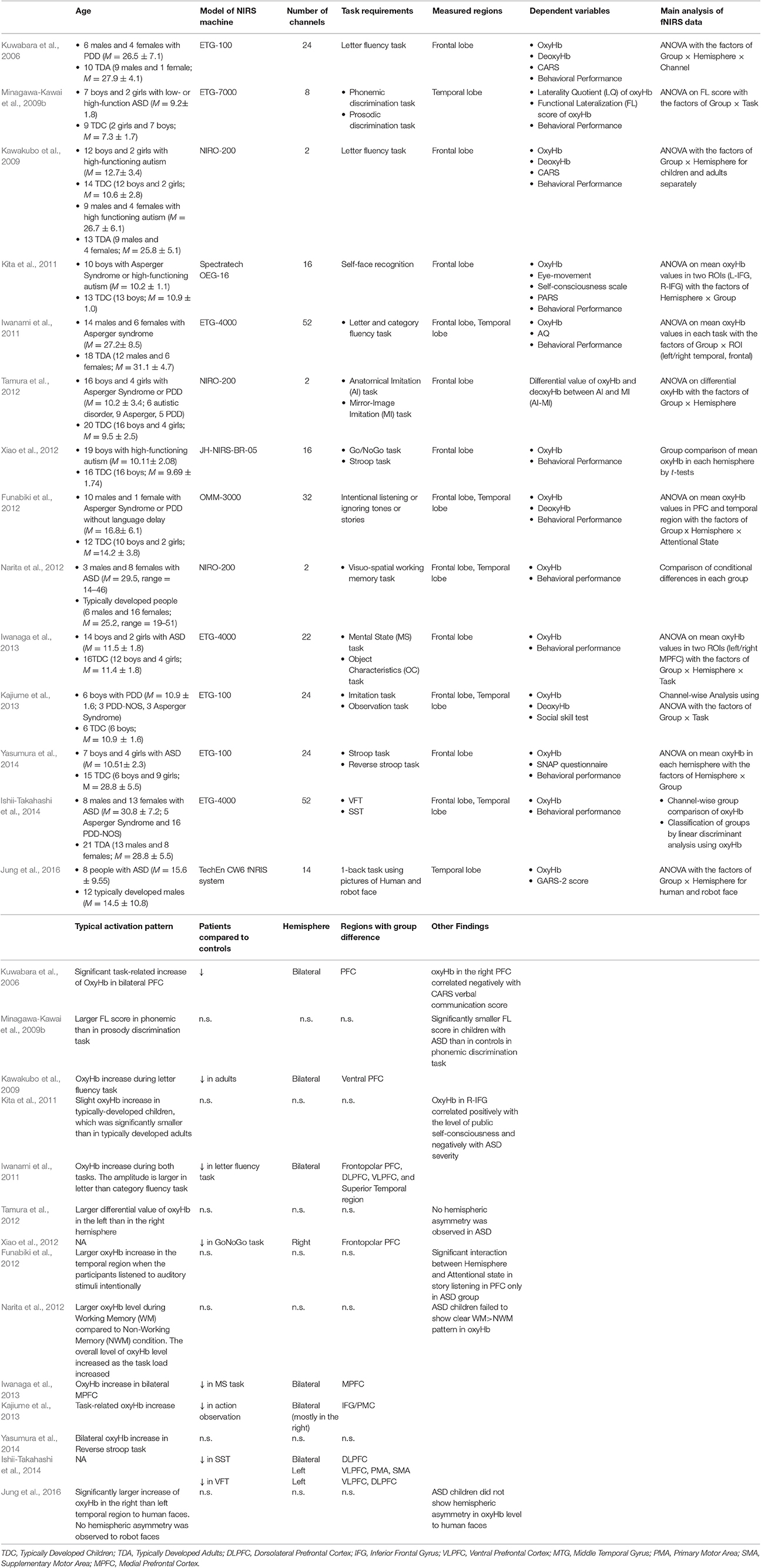
Table 2. The details of the main fNIRS studies on people with ASD explained in the present mini-review: only the results of group comparison with matched-controls are shown.
Several studies report reduced lateralization in neural function in people with ASD. For example, Minagawa-Kawai et al. (2009b) reported weaker leftward-lateralization of oxyHb increases in Wernicke areas when children with ASD engaged in a phonemic discrimination task, although they did not report the results of direct group-comparisons of task-related oxyHb changes. Likewise, Jung et al. (2016) reported that people with ASD failed to show rightward-lateralization of oxyHb increases in the posterior temporal region in response to human faces as was observed in the matched control group.
One potential reason for such inconsistency among previous studies is the symptomatic heterogeneity of ASD. ASD has several sub-groups that differ in symptomatic profiles and cognitive-emotional ability (Lenroot and Yeung, 2013). The left-hemisphere theory of ASD was originally proposed for individuals with Kanner's autism with language delay (McCann, 1982). However, most fNIRS studies have recruited people with high-functioning autism or Asperger Syndrome (Kawakubo et al., 2009; Iwanami et al., 2011; Kita et al., 2011; Xiao et al., 2012; Iwanaga et al., 2013; Yasumura et al., 2014), possibly due to the task requirements, with rare exceptions (Minagawa-Kawai et al., 2009b). Considering this, it is possible that evidence supporting a clearer pattern of lateralization can be obtained for specific sub-groups.
General Discussion
Existing fNIRS studies generally support the notion of the right-lateralization in atypical function in children with ADHD. The use of fNIRS for the clinical examination of children is promising, especially because the exclusion rate for fNIRS measurement is reported to be much lower than that for fMRI (Nagashima et al., 2014b). This potential has been gainfully exploited by Monden et al. (2012, 2015), who assessed the efficacy of a pharmacological intervention in children with ADHD using oxyHb increases in the right PFC as an indicator (see also, Nagashima et al., 2014a,b,c). Future research should estimate the sensitivity/specificity of right PFC activation as a biomarker of ADHD (Monden et al., 2015) and investigate whether the rightward-lateralization in atypical activation is uniquely linked to ADHD. We did not find a clear pattern of leftward-lateralization in atypical function for people with ASD. As noted above, this is partly because of the heterogeneity of people with ASD.
There remain several unresolved issues important for the further development of research on the lateralization in atypical neural function. The first is the establishment of a standard analytic method. As summarized in the tables, the analytic approach for multi-channel fNIRS data can be classified into two groups. One is the region-of-interest (ROI) approach, in which neighboring channels are grouped into single ROI and the averaged levels of oxyHb in channels within ROI are analyzed as the main indicators of neural activation. In this approach, corresponding channels in the left and right hemisphere are usually integrated into left/right ROI. The other approach is channel-wise analysis, in which a primary statistical test is conducted for each channel. It is unclear at this point which of these two approaches is more advantageous for detecting lateralized patterns in atypical activation. Channel-wise analysis is more sensitive to highly localized group-differences than the ROI approach, and thus might be more suitable for detecting signs of lateralization in atypical function. The main problem of the channel-wise approach is how to set the significance threshold. Apparently, a large number of statistical tests leads to inflation of the false-positive rate, while a conventional method for adjusting the threshold, e.g., Bonferroni's procedure, is sometimes too stringent.
The second issue also relates to the analytic procedure. There are several problems in the group comparisons of fNIRS results. First, due to morphological variations in cortical structure, the location and depth of the cortical region through which the infrared light passes might differ between people with and without developmental disorders. Second, it is often noted that children with ADHD/ASD show larger bodily and facial movements than matched controls during experimental tasks, which might introduce group-differences in the level of artifacts and consequently influence the results. Especially problematic is the artifact of skin blood perfusion accompanying facial muscle contractions (Takahashi et al., 2011; Seiyama et al., 2016). To overcome these problems inherent in group-comparisons of fNIRS signals is surely an important agenda for future research.
The third point is the scarcity of fNIRS studies on resting state activation (Medvedev, 2014). Of particular relevance, one of the strongest pieces of evidence for the left-hemisphere theory of ASD comes from a resting state activation study (Cardinale et al., 2013). Thus, more research should focus on the patterns of lateralization of oxy-/deoxy-Hb alteration in the resting state. One of the most popular approaches to characterizing resting state activity is the analysis of inter-region functional connectivity. Several fNIRS studies have tried to characterize neural function in developmental disorders (Kikuchi et al., 2013; Zhu et al., 2015; Li and Yu, 2016; Li et al., 2016), and interestingly, several of them found lateralized patterns of atypical connectivity in the patient group (Zhu et al., 2015). We did not review fNIRS studies of functional connectivity in people with ADHD or ASD, as the analysis procedures vary greatly between studies and the number of eligible studies is too small to draw any coherent conclusions. However, considering the rapid development of this field of research, our knowledge of the lateralization in atypical function is further enriched by this novel approach.
The fourth is the potential confound of medication. The number of studies recruiting only drug-naïve patients is relatively few and participants in the patient group are taking various kinds of medications in majority of the studies. Fuethremore, several fNIRS studies reviewed above have shown that short-term administration of drugs such as methylphenidate changed the pattern of cortical activation in children with ADHD (Nagashima et al., 2014a,b,c; Monden et al., 2015). On the basis of these, more studies recruiting only non-medicated patients are needed to clarify the precise nature of atypicality in neural function.
Conclusion
In this mini-review, we gave a brief overview of the findings of fNIRS studies about lateralization in atypical neural function in people with ADHD and ASD. The existing studies generally support rightward-lateralization in atypical function in children with ADHD. At the same time, we did not find clear pattern of the leftward-lateralization in atypical function for people with ASD. Nevertheless, lateralization in atypical neural function might have been obscured by factors such as sample heterogeneity and particular method of analysis.
Author Contributions
HD conceived this study. HD and KS wrote the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank Dr Ryoichiro Iwanaga for his comments on the early version of this manuscript. This work was partially supported by JSPS KAKENHI Grant-in-Aid for Scientific Research (C) (Grant No. 26461769) to HD.
References
Adorni, R., Gatti, A., Brugnera, A., Sakatani, K., and Compare, A. (2016). Could fNIRS promote neuroscience approach in clinical psychology? Front. Psychol. 7:456. doi: 10.3389/fpsyg.2016.00456
Anderson, J. S., Druzgal, T. J., Froehlich, A., DuBray, M. B., Lange, N., Alexander, A. L., et al. (2011). Decreased interhemispheric functional connectivity in autism. Cereb. Cortex 21, 1134–1146. doi: 10.1093/cercor/bhq190
APA (2013). Diagnostic and Statistical Manual of Mental Disorders, 5th Edn. Washington, DC: American Psychiatric Publishing.
Araki, A., Ikegami, M., Okayama, A., Matsumoto, N., Takahashi, S., Azuma, H., et al. (2015). Improved prefrontal activity in AD/HD children treated with atomoxetine: a NIRS study. Brain Dev. 37, 76–87. doi: 10.1016/j.braindev.2014.03.011
Balconi, M., Grippa, E., and Vanutelli, M. E. (2015). What hemodynamic (fNIRS), electrophysiological (EEG) and autonomic integrated measures can tell us about emotional processing. Brain Cogn. 95, 67–76. doi: 10.1016/j.bandc.2015.02.001
Cardinale, R. C., Shih, P., Fishman, I., Ford, L. M., and Müller, R. A. (2013). Pervasive rightward asymmetry shifts of functional networks in autism spectrum disorder. JAMA Psychiatry 70, 975–982. doi: 10.1001/jamapsychiatry.2013.382
Crow, T. J. (2000). Schizophrenia as the price that Homo sapiens pays for language: a resolution of the central paradox in the origin of the species. Brain Res. Rev. 31, 118–129. doi: 10.1016/S0165-0173(99)00029-6
De Fossé, L., Hodge, S. M., Makris, N., Kennedy, D. N., Caviness, V. S. Jr., McGrath, L., et al. (2004). Language-association cortex asymmetry in autism and specific language impairment. Ann. Neurol. 56, 757–766. doi: 10.1002/ana.20275
Doi, H., Nishitani, S., and Shinohara, K. (2013). NIRS as a tool for assaying emotional function in the prefrontal cortex. Front. Hum. Neurosci. 7:770. doi: 10.3389/fnhum.2013.00770
Ehlis, A. C., Bähne, C. G., Jacob, C. P., Herrmann, M. J., and Fallgatter, A. J. (2008). Reduced lateral prefrontal activation in adult patients with attention-deficit/hyperactivity disorder (ADHD) during a working memory task: a functional near-infrared spectroscopy (fNIRS) study. J. Psychiatr. Res. 42, 1060–1067. doi: 10.1016/j.jpsychires.2007.11.011
Ehlis, A.-C., Schneider, S., Dresler, T., and Fallgatter, A. J. (2014). Application of functional near-infrared spectroscopy in psychiatry. Neuroimage 85, 478–488. doi: 10.1016/j.neuroimage.2013.03.067
Floris, D. L., Lai, M. C., Auer, T., Lombardo, M. V., Ecker, C., Chakrabarti, B., et al. (2016). Atypically rightward cerebral asymmetry in male adults with autism stratifies individuals with and without language delay. Hum. Brain Mapp. 37, 230–253. doi: 10.1002/hbm.23023
Funabiki, Y., Murai, T., and Toichi, M. (2012). Cortical activation during attention to sound in autism spectrum disorders. Res. Dev. Disabil. 33, 518–524. doi: 10.1016/j.ridd.2011.10.016
Hale, T. S., Loo, S. K., Zaidel, E., Hanada, G., MacIon, J., and Smalley, S. L. (2009). Rethinking a right hemisphere deficit in ADHD. J. Atten. Disord. 13, 3–17. doi: 10.1177/1087054708323005
Hervé, P. Y., Zago, L., Petit, L., Mazoyer, B., and Tzourio-Mazoyer, N. (2013). Revisiting human hemispheric specialization with neuroimaging. Trends Cogn. Sci. 17, 80. doi: 10.1016/j.tics.2012.12.004
Hoshi, Y., and Tamura, M. (1993). Dynamic multichannel near-infrared optical imaging of human brain activity. J. Appl. Physiol. 75, 1842–1846.
Ichikawa, H., Nakato, E., Kanazawa, S., Shimamura, K., Sakuta, Y., Sakuta, R., et al. (2014). Hemodynamic response of children with attention-deficit and hyperactive disorder (ADHD) to emotional facial expressions. Neuropsychologia 63, 51–58. doi: 10.1016/j.neuropsychologia.2014.08.010
Inoue, Y., Sakihara, K., Gunji, A., Ozawa, H., Kimiya, S., Shinoda, H., et al. (2012). Reduced prefrontal hemodynamic response in children with ADHD during the Go/NoGo task: a NIRS study. Neuroreport 23, 55–60. doi: 10.1097/WNR.0b013e32834e664c
Ishii-Takahashi, A., Takizawa, R., Nishimura, Y., Kawakubo, Y., Kuwabara, H., Matsubayashi, J., et al. (2014). Prefrontal activation during inhibitory control measured by near-infrared spectroscopy for differentiating between autism spectrum disorders and attention deficit hyperactivity disorder in adults. NeuroImage Clin. 4, 53–63. doi: 10.1016/j.nicl.2013.10.002
Ishii-Takahashi, A., Takizawa, R., Nishimura, Y., Kawakubo, Y., Hamada, K., Okuhata, S., et al. (2015). Neuroimaging-aided prediction of the effect of methylphenidate in children with attention-deficit hyperactivity disorder: a randomized controlled trial. Neuropsychopharmacology 40, 4676–4685. doi: 10.1038/npp.2015.128
Iwanaga, R., Tanaka, G., Nakane, H., Honda, S., Imamura, A., and Ozawa, H. (2013). Usefulness of near-infrared spectroscopy to detect brain dysfunction in children with autism spectrum disorder when inferring the mental state of others. Psychiatry Clin. Neurosci. 67, 203–209. doi: 10.1111/pcn.12052
Iwanami, A., Okajima, Y., Ota, H., Tani, M., Yamada, T., Hashimoro, R., et al. (2011). Task dependent prefrontal dysfunction in persons with Asperger's disorder investigated with multi-channel near-infrared spectroscopy. Res. Autism Spectr. Disord. 5, 1187–1193. doi: 10.1016/j.rasd.2011.01.005
Jourdan Moser, S., Cutini, S., Weber, P., and Schroeter, M. L. (2009). Right prefrontal brain activation due to Stroop interference is altered in attention-deficit hyperactivity disorder - a functional near-infrared spectroscopy study. Psychiatry Res. 173, 190–195. doi: 10.1016/j.pscychresns.2008.10.003
Jung, C. E., Strother, L., Feil-Seifer, D. J., and Hutsler, J. J. (2016). Atypical asymmetry for processing human and robot faces in autism revealed by fNIRS. PLoS ONE 11:e0158804. doi: 10.1371/journal.pone.0158804
Kajiume, A., Aoyama-Setoyama, S., Saito-Hori, Y., Ishikawa, N., and Kobayashi, M. (2013). Reduced brain activation during imitation and observation of others in children with pervasive developmental disorder: a pilot study. Behav. Brain Funct. 9:21. doi: 10.1186/1744-9081-9-21
Kato, T., Kamei, A., Takashima, S., and Ozaki, T. (1993). Human visual cortical function during photic stimulation monitoring by means of near-infrared spectroscopy. J. Cereb. Blood Flow Metab. 13, 516–520. doi: 10.1038/jcbfm.1993.66
Kawakubo, Y., Kuwabara, H., Watanabe, K.-I., Minowa, M., Someya, T., Minowa, I., et al. (2009). Impaired prefrontal hemodynamic maturationin autism and unaffected siblings. PLoS ONE 4:e6881. doi: 10.1371/journal.pone.0006881
Kikuchi, M., Yoshimura, Y., Shitamichi, K., Ueno, S., Hiraishi, H., Munesue, T., et al. (2013). Anterior prefrontal hemodynamic connectivity in conscious 3- to 7-year-old children with typical development and autism spectrum disorder. PLoS ONE 8:e56087. doi: 10.1371/journal.pone.0056087
Kita, Y., Gunji, A., Inoue, Y., Goto, T., Sakihara, K., Kaga, M., et al. (2011). Self-face recognition in children with autism spectrum disorders: a near-infrared spectroscopy study. Brain Dev. 33, 494–503. doi: 10.1016/j.braindev.2010.11.007
Klimkeit, E. I., and Bradshaw, J. L. (2006). Anomalous lateralisation in neurodevelopmental disorders. Cortex 42, 113–116. doi: 10.1016/S0010-9452(08)70334-4
Köchel, A., Schöngaßner, F., Feierl-Gsodam, S., and Schienle, A. (2015). Processing of affective prosody in boys suffering from attention deficit hyperactivity disorder: a near-infrared spectroscopy study. Soc. Neurosci. 10, 583–591. doi: 10.1080/17470919.2015.1017111
Koike, S., Nishimura, Y., Takizawa, R., Yahata, N., and Kasai, K. (2013). Near-infrared spectroscopy in schizophrenia: a possible biomarker for predicting clinical outcome and treatment response. Front. Psychiatry 4:145. doi: 10.3389/fpsyt.2013.00145
Kuwabara, H., Kasai, K., Takizawa, R., Kawakubo, Y., Yamasue, H., Rogers, M. A., et al. (2006). Decreased prefrontal activation during letter fluency task in adults with pervasive developmental disorders: a near-infrared spectroscopy study. Behav. Brain Res. 172, 272–277. doi: 10.1016/j.bbr.2006.05.020
Lenroot, R. K., and Yeung, P. K. (2013). Heterogeneity within autism spectrum disorders: what have we learned from neuroimaging studies? Front. Hum. Neurosci. 7:733. doi: 10.3389/fnhum.2013.00733
Li, J., Qiu, L., Xu, L., Pedapati, E. V., Erickson, C. A., and Sunar, U. (2016). Characterization of autism spectrum disorder with spontaneous hemodynamic activity. Biomed. Opt. Express 7, 3871–3881. doi: 10.1364/BOE.7.003871
Li, Y., and Yu, D. (2016). Weak network efficiency in young children with Autism Spectrum Disorder: evidence from a functional near-infrared spectroscopy study. Brain Cogn. 108, 47–55. doi: 10.1016/j.bandc.2016.07.006
McCann, B. S. (1982). Hemispheric asymmetries and early infantile autism. J. Autism Dev. Disord. 11, 401–411. doi: 10.1007/BF01531615
Medvedev, A. V. (2014). Does the resting state connectivity have hemispheric asymmetry? A near-infrared spectroscopy study. Neuroimage 85, 400–407. doi: 10.1016/j.neuroimage.2013.05.092
Minagawa-Kawai, Y., Naoi, N., Kikuchi, N., Yamamoto, J., Nakamura, K., and Kojima, S. (2009b). Cerebral laterality for phonemic and prosodic cue decoding in children with autism. Neuroreport 20, 1219–1224. doi: 10.1097/WNR.0b013e32832fa65f
Minagawa-Kawai, Y., Naoi, N., and Kojima, S. (2009a). A New Approach to Functional Neuroimaging: Near-Infrared Spectroscopy (NIRS). Tokyo: Keio University Press.
Monden, Y., Dan, H., Nagashima, M., Dan, I., Tsuzuki, D., Kyutoku, Y., et al. (2012). Right prefrontal activation as a neuro-functional biomarker for monitoring acute effects of methylphenidate in ADHD children: an fNIRS study. Neuroimage Clin. 1, 131–140. doi: 10.1016/j.nicl.2012.10.001
Monden, Y., Dan, I., Nagashima, M., Dan, H., Uga, M., Ikeda, T., et al. (2015). Individual classification of ADHD children by right prefrontal hemodynamic responses during a go/no-go task as assessed by fNIRS. Neuroimage Clin. 9, 1–12. doi: 10.1016/j.nicl.2015.06.011
Nagashima, M., Monden, Y., Dan, I., Dan, H., Mizutani, T., Tsuzuki, D., et al. (2014c). Neuropharmacological effect of atomoxetine on attention network in children with attention deficit hyperactivity disorder during oddball paradigms as assessed using functional near-infrared spectroscopy. Neurophotonics 1, 14057R. doi: 10.1117/1.NPh.1.2.025007
Nagashima, M., Monden, Y., Dan, I., Dan, H., Tsuzuki, D., Mizutani, T., et al. (2014b). Neuropharmacological effect of methylphenidate on attention network in children with attention deficit hyperactivity disorder during oddball paradigms as assessed using functional near-infrared spectroscopy. Neurophotonics 1:015001. doi: 10.1117/1.NPh.1.1.015001
Nagashima, M., Monden, Y., Dan, I., Dan, I., Dan, H., Tsuzuki, D., et al. (2014a). Acute neuropharmacological effects of atomoxetine on inhibitory control in ADHD children: a fNIRS study. Neuroimage Clin. 6, 192–201. doi: 10.1016/j.nicl.2014.09.001
Narita, N., Saotome, A., Higuchi, H., Masaaki, N., Tazoe, M., and Sakatani, K. (2012). Impaired prefrontal cortical response by switching stimuli in autism spectrum disorders. J. Pediatr. Neurol. 10, 87–94. doi: 10.3233/JPN-2012-0541
Negoro, H., Sawada, M., Iida, J., Ota, T., Tanaka, S., and Kishimoto, T. (2010). Prefrontal dysfunction in attention-deficit/hyperactivity disorder as measured by near-infrared spectroscopy. Child Psychiatry Hum. Dev. 41, 193–203. doi: 10.1007/s10578-009-0160-y
Pietrzak, R. H., Mollica, C. M., Maruff, P., and Snyder, P. J. (2006). Cognitive effects of immediate-release methylphenidate in children with attention-deficit/hyperactivity disorder. Neurosci. Biobehav. Rev. 30, 1225–1245. doi: 10.1016/j.neubiorev.2006.10.002
Safren, S. A., Sprich, S., Mimiaga, M. J., Surman, C., Knouse, L., Groves, M., et al. (2010). Cognitive behavioral therapy vs relaxation with educational support for medication-treated adults with ADHD and persistent symptoms: a randomized controlled trial. JAMA 304, 875–880. doi: 10.1001/jama.2010.1192
Schecklmann, M., Ehlis, A.-C., Plichta, M. M., Dresler, T., Heine, M., Boreatti-Hümmer, A., et al. (2012). Working Memory and Response Inhibition as One Integral Phenotype of Adult ADHD? A behavioral and imaging correlational investigation. J. Atten. Disord. 17, 470–482. doi: 10.1177/1087054711429702
Schecklmann, M., Ehlis, A.-C., Plichta, M. M., Romanos, J., Heine, M., Boreatti-Hümmer, A., et al. (2009). Diminished prefrontal oxygenation with normal and above-average verbal fluency performance in adult ADHD. J. Psychiatr. Res. 43, 98–106. doi: 10.1016/j.jpsychires.2008.02.005
Schecklmann, M., Romanos, M., Bretscher, F., Plichta, M. M., Warnke, A., and Fallgatter, A. J. (2010). Prefrontal oxygenation during working memory in ADHD. J. Psychiatr. Res. 44, 621–628. doi: 10.1016/j.jpsychires.2009.11.018
Schecklmann, M., Schaldecker, M., Aucktor, S., Brast, J., Kirchgässner, K., Mühlberger, A., et al. (2011a). Effects of methylphenidate on olfaction and frontal and temporal brain oxygenation in children with ADHD. J. Psychiatr. Res. 45, 1463–1470. doi: 10.1016/j.jpsychires.2011.05.011
Schecklmann, M., Schenk, E., Maisch, A., Kreiker, S., Jacob, C., Warnke, A., et al. (2011b). Altered frontal and temporal brain function during olfactory stimulation in adult attention-deficit/hyperactivity disorder. Neuropsychobiology 63, 66–76. doi: 10.1159/000323448
Seiyama, A., Higaki, K., Takeuchi, N., Uehara, M., and Takayama, N. (2016). Estimation of skin blood flow artefacts in nirs signals during a verbal fluency task. Adv. Exp. Med. Biol. 876, 327–334. doi: 10.1007/978-1-4939-3023-4_41
Silk, T. J., Vilgis, V., Adamson, C., Chen, J., Smit, L., Vance, A., et al. (2016). Abnormal asymmetry in frontostriatal white matter in children with attention deficit hyperactivity disorder. Brain Imaging Behav. 10, 1080–1089. doi: 10.1007/s11682-015-9470-9
Song, A. W., Huettel, S. A., and McCarthy, G. (2006). “Functional neuroimaging: basic principles of functional MRI,” in Handbook of Functional Neuroimaging of Cognition, eds R. Cabeza and A. Kingstone (Cambridge, MA: The MIT Press), 21–52.
Stefanatos, G. A., and Wasserstein, J. (2001). Attention deficit/hyperactivity disorder as a right hemisphere syndrome: selective literature review and detailed neuropsychological case studies. Ann. N. Y. Acad. Sci. 931, 172–195. doi: 10.1111/j.1749-6632.2001.tb05779.x
Takahashi, T., Takikawa, Y., Kawagoe, R., Shibuya, S., Iwano, T., and Kitazawa, S. (2011). Influence of skin blood flow on near-infrared spectroscopy signals measured on the forehead during a verbal fluency task. Neuroimage 57, 991–1002. doi: 10.1016/j.neuroimage.2011.05.012
Tamura, R., Kitamura, H., Endo, T., Abe, R., and Someya, T. (2012). Decreased leftward bias of prefrontal activity in autism spectrum disorder revealed by functional near-infrared spectroscopy. Psychiatry Res. 203, 237–224. doi: 10.1016/j.pscychresns.2011.12.008
Thomas, R., Sanders, S., Doust, J., Beller, E., and Glasziou, P. (2015). Prevalence of attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. Pediatrics 135, e994–e1001. doi: 10.1542/peds.2014-3482
Toga, A. W., and Thompson, P. M. (2003). Mapping brain asymmetry. Nat. Rev. Neurosci. 4, 37–48. doi: 10.1038/nrn1009
Tsujimoto, S., Yasumura, A., Yamashita, Y., Torii, M., Kaga, M., and Inagaki, M. (2013). Increased prefrontal oxygenation related to distractor-resistant working memory in children with attention-deficit/hyperactivity disorder (ADHD) Child Psychiatry Hum. Dev. 44, 678–688. doi: 10.1007/s10578-013-0361-2
Valera, E. M., Faraone, S. V., Murray, K. E., and Seidman, L. J. (2007). Meta-Analysis of structural imaging findings in attention-deficit/hyperactivity disorder. Biol. Psychiatry 61, 1361–1369. doi: 10.1016/j.biopsych.2006.06.011
Weber, P., Lütschg, J., and Fahnenstich, H. (2005). Cerebral hemodynamic changes in response to an executive function task in children with attention-deficit hyperactivity disorder measured by near-infrared spectroscopy. J. Dev. Behav. Pediatr. 26, 105–111. doi: 10.1097/00004703-200504000-00005
Xiao, T., Xiao, Z., Ke, X., Hong, S., Yang, H., Su, Y., et al. (2012). Response inhibition impairment in high functioning autism and attention deficit hyperactivity disorder: evidence from near-infrared spectroscopy data. PLoS ONE 7:e46569. doi: 10.1371/journal.pone.0046569
Yasumura, A., Kokubo, N., Yamamoto, H., Yasumura, Y., Nakagawa, E., Kaga, M., et al. (2014). Neurobehavioral and hemodynamic evaluation of Stroop and reverse Stroop interference in children with attention-deficit/hyperactivity disorder. Brain Dev. 36, 97–106. doi: 10.1016/j.braindev.2013.01.005
Yasumura, A., Yamamoto, H., Yasumura, Y., Moriguchi, Y., Hiraki, K., Nakagawa, E., et al. (2015). Cognitive shifting in children with attention-deficit hyperactivity disorder: a near infrared spectroscopy study. Afr. J. Psychiatry 18:196. doi: 10.4172/Psychiatry.1000196
Keywords: fNIRS, hemispheric asymmetry, ADHD, ASD, prefrontal cortex, latealization
Citation: Doi H and Shinohara K (2017) fNIRS Studies on Hemispheric Asymmetry in Atypical Neural Function in Developmental Disorders. Front. Hum. Neurosci. 11:137. doi: 10.3389/fnhum.2017.00137
Received: 07 November 2016; Accepted: 09 March 2017;
Published: 12 April 2017.
Edited by:
Leonid Perlovsky, Harvard University and Air Force Research Laboratory, USACopyright © 2017 Doi and Shinohara. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kazuyuki Shinohara, kazuyuki@nagasaki-u.ac.jp
 Hirokazu Doi
Hirokazu Doi Kazuyuki Shinohara
Kazuyuki Shinohara