The Role of Brain-Derived Neurotrophic Factor Signaling in Central Nervous System Disease Pathogenesis
- 1Department of Neuroscience, Institute of Chinese Medicine, Heilongjiang University of Chinese Medicine, Harbin, China
- 2Department of Veterinary Medicine, College of Agriculture, Hainan University, Haikou, China
Recent studies have found abnormal levels of brain-derived neurotrophic factor (BDNF) in a variety of central nervous system (CNS) diseases (e.g., stroke, depression, anxiety, Alzheimer’s disease, and Parkinson’s disease). This suggests that BDNF may be involved in the pathogenesis of these diseases. Moreover, regulating BDNF signaling may represent a potential treatment for such diseases. With reference to recent research papers in related fields, this article reviews the production and regulation of BDNF in CNS and the role of BDNF signaling disorders in these diseases. A brief introduction of the clinical application status of BDNF is also provided.
Introduction
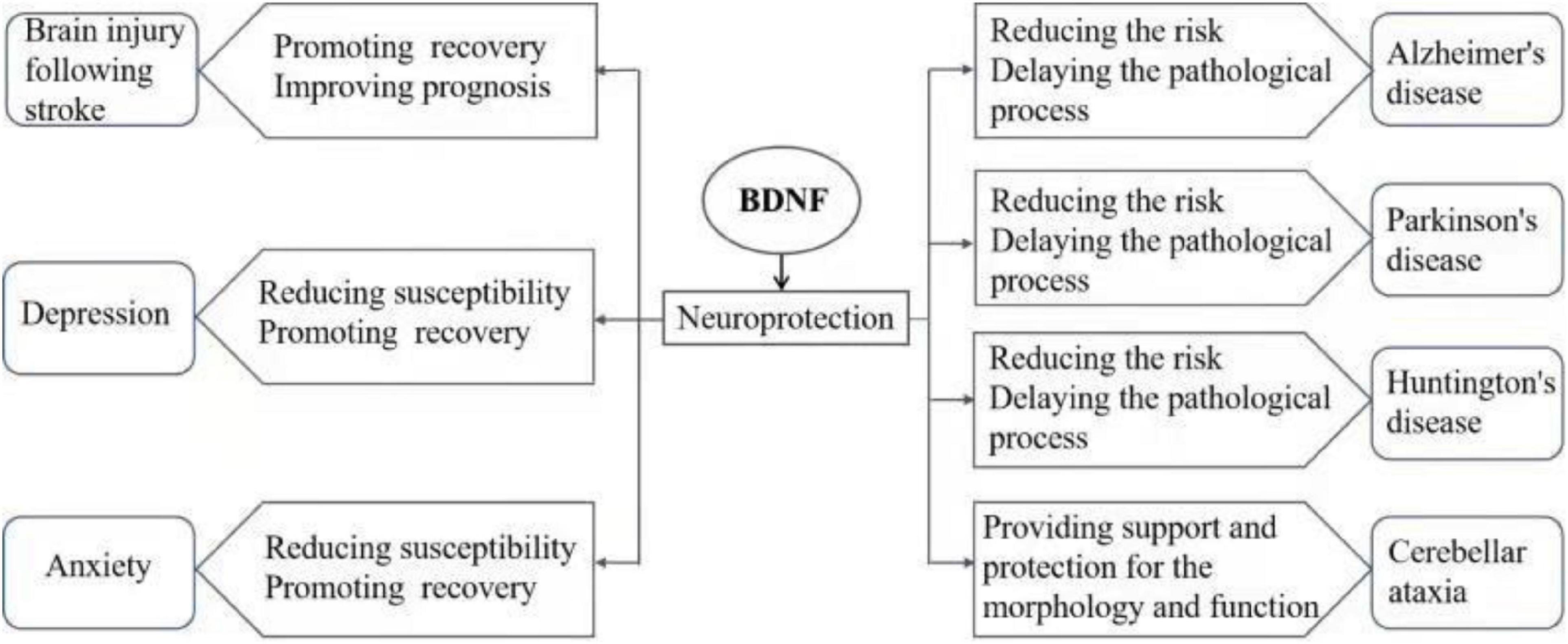
Brain-derived neurotrophic factor (BDNF) is a small molecule dimer protein, and the main member of the neurotrophic protein family in the brain (Song et al., 2017). BDNF is widely expressed in the central nervous system (CNS), endocrine system, bone and cartilage tissue, as is particularly highly concentrated within the hippocampus and cortex in the brain. BDNF plays an important role in the survival and proliferation and differentiation of neurons and glial cells, axon growth, synapse formation, and regulation of synaptic transmission and plasticity (Nagahara and Tuszynski, 2011; Kowiański et al., 2018). Conversely, disorder of BDNF signaling induces dysfunctions in CNS. Abnormal BDNF signaling has been found in many CNS diseases, and is considered to be involved in the pathological process of the disorders, affecting the occurrence, development and prognosis. Currently, researches on mental and neurological disease (e.g., stroke, mood disorders, and neurodegenerative diseases) found that abnormal BDNF signaling is involved in the diseases. In this article, the role of BDNF in the pathogenesis of a series of CNS diseases (including brain injury after stroke, depression, anxiety disorder, and neurodegenerative diseases, such as Alzheimer’s disease (AD), Parkinson’s disease (PD), Huntington’s disease (HD), and cerebellar ataxia) was reviewed (Figure 1), and the current status of research in the clinical application of BDNF and proposed future research directions are discussed.
Sources and Physiological Functions of Brain-Derived Neurotrophic Factor
Molecular Structure of Brain-Derived Neurotrophic Factor
Brain-derived neurotrophic factor represents the most abundant and widely studied neurotrophic factor in the mammalian nervous system. Barde et al. (1982) first isolated and purified an alkaline protein from porcine cerebrospinal fluid, which had a very similar amino acid sequence and associated biological activity with the known nerve growth factor (NGF) structure. Consequently, BDNF is collectively known as the “neurotrophin family” together with NT-3, NT-4, and NT-5, which were later cloned. The BDNF gene is located in the p13–14 region of chromosome 11, with a full length of approximately 70 kb. This gene is composed of 11 exons at the 5′ end and contains 9 functional promoters specific for tissues and brain regions (Pruunsild et al., 2007). The BDNF molecular monomer is a secretory polypeptide composed of 119 amino acid residues. The isoelectric point of the protein is 9.99, and the molecular mass is 13.15 KDa. BDNF is primarily composed of a β folding and random coil secondary structure, contains three disulfide bonds, and exists in the form of dimer in vivo.
Production, Existence, and Physiological Function of Brain-Derived Neurotrophic Factor
Brain-derived neurotrophic factor is produced by neurons and glial cells in the mammalian brain and is mainly distributed throughout key brain regions (e.g., the cortex, hippocampus, and cerebellum). Astrocytes are also an important source of BDNF, and BDNF released from astrocytes has been demonstrated to exhibit neuroprotective and neuroregenerative effects, such as promotion of neogenesis and development of glial cells (Quesseveur et al., 2013). In addition, astrocytes promote microglia to express BDNF by releasing cytokines, inducing microglia transformation, and regulating their function (Clarke and Barres, 2013; Louveau et al., 2015). In several pathological conditions, glial cell-derived BDNF is involved in the recovery of disease and injury repair processes.
Brain-derived neurotrophic factor exists as two forms: precursor form of BDNF (pro-BDNF) and mature form of BDNF (m-BDNF). BDNF is first translated into the pro-BDNF in the endoplasmic reticulum. Pro-BDNF is subsequently cleaved by serine proteases in the Golgi and endoplasmic reticulum to form m-BDNF (Zhao et al., 2022). Early studies showed that only m-BDNF is biologically active, while pro-BDNF as an intermediate does not exert biological function. However, recent studies confirmed that pro-BDNF can exist as a precursor form of BDNF, and also can be directly secreted from synapses extracellularly exert a variety of biological effects. Both pro-BDNF and m-BDNF presenting in neurons are released following cell membrane depolarization. In addition, there is a dynamic balance between the different forms of BDNF, and the ratio of Pro-BDNF to m-BDNF varies between specific stages and regions of brain development. Although pro-BDNF exists at higher concentrations during the early stages of brain development, m-BDNF is dominant during the mature stage. Therefore, the ratio of pro-BDNF to m-BDNF during early development is considered to represent an important factor for the regulation of brain function (Zhou et al., 2013; Qiao et al., 2017; Li et al., 2019). In contrast, m-BDNF plays an essential role in neuroprotection and synaptic plasticity following maturation.
Brain-derived neurotrophic factor binds to two types of receptors: high-affinity receptor tyrosine kinase receptor B (TrkB) and low-affinity neurotrophin factor receptor (p75NTR). BDNF binds to the receptors to activate a series of downstream signaling pathways to promote neurogenesis, neuronal growth and survival, enhance synaptic plasticity, and exert neurotrophic effects (Andero et al., 2014). BDNF binding to TrkB promotes astrocyte development. It was found that the TrkB gene knockout mice showed an incomplete astrocyte morphology, which was not able to support normal synaptic function (Holt et al., 2019). Furthermore, BDNF/TrkB can mediate hippocampal plasticity and promote the survival and integration of the hippocampal newborn neurons (Li et al., 2008).
However, BDNF binds to p75NTR may promote apoptosis and accelerate pathological process. It has been reported that the increased expression of p75NTR in the brain can reduce the neuroprotective effects of BDNF mentioned above, and the expression of p75NTR is significantly increased in some pathological conditions associated with the decline in brain function including learning and memory (Chakravarthy et al., 2012). This suggests that BDNF is bound or reduced when p75NTR is highly expressed, resulting in the competitive inhibition of TrkB (Brito et al., 2013). In addition, it has reported that pro-BDNF mainly binding to p75NTR plays a role in neuronal apoptosis and promotes synaptic long-term depression (Woo et al., 2005). On the other hand, p75NTR expression was significantly elevated in some conditions of decreased brain function. This raises the question “why the nervous system induces a pro-apoptotic molecule to respond to damage” (Ibáñez and Simi, 2012), and therefore, it is argued that p75NTR can promote apoptosis after injury to eliminate damaged cells, minimize inflammation, and maintain suitable environment for the cells (Meeker and Williams, 2015). The detailed roles of p75NTR remains to be discovered in the future.
Brain-Derived Neurotrophic Factor and Brain Injury Following Stroke
Stroke is a type of acute cerebrovascular disease involving a group of diseases that damage brain tissue when blood vessels in the brain burst or become blocked, which prevent blood flow to the brain. Following a stroke, there is increased risk of abnormal remodeling of the hippocampus, decreased neurogenesis and neuronal apoptosis, as well as neuron damage, which increases the risk of depression. The recovery of post-stroke injury was found to be promoted by an increase in BDNF levels (Chen et al., 2015). Furthermore, Hsu et al. (2020) found that the regulation of endogenous BDNF production could alleviate brain injury following stroke. At the same time, exogenous BDNF has been found to reduce the infarct size caused by local ischemia, protect neurons, as well as promote neuronal survival and differentiation (Kurozumi et al., 2004). BDNF improves cognitive function by improving neuronal plasticity and increasing acetylcholinesterase activity. Indeed, an intravenous injection of BDNF has been shown to enhance cognitive recovery and stimulate neurogenesis following a stroke (Schäbitz et al., 2007). In addition, BDNF supports the development, differentiation, growth, and regeneration of 5-hydroxy tryptamine (5-HT) and dopamine (DA) neurons (Levivier et al., 1995; Mamounas et al., 2000), and also promotes the generation and release of neurotransmitters and improves neurological function. The expression of BDNF was found to be triggered by brain injury as part of the neuroprotective response following a stroke (Kokaia et al., 1998). Moreover, pro-BDNF plays a role in apoptosis following its binding to the p75NTR receptor, which aggravates post-stroke depression (PSD) (Yang et al., 2021). These results indicate that regulation of the BDNF signaling pathway has the potential to treat brain injury after a stroke.
Brain-Derived Neurotrophic Factor and Common Mood Disorders
Brain-Derived Neurotrophic Factor and Depression
Depression represents one of the most common emotional disorders and is characterized by an abnormal and persistent low mood. Depression is also accompanied by cognitive and physical changes, including anhedonia, slow response, and loss of appetite. Moreover, depression is associated with both a high recurrence and suicide rate. The neurotrophic factor hypothesis holds that a decrease in neurotrophic factors is the pathological basis of depression (Levy et al., 2018). Thus, increasing or restoring the content of BDNF can help reverse brain damage and promote the expression of nutritional proteins, which is an antidepressant effect. The study by Saarelainen et al. (2003) confirmed the role of endogenous BDNF, which was found increase of the release of BDNF and BDNF-TrkB signal transduction in the brain following the administration of antidepressants. Infusion of BDNF into the midbrain of mice with learned helplessness was found to improve depressive-like behavior; however, its antidepressant effect disappeared following the administration with a TrkB inhibitor (Siuciak et al., 1997; Shirayama et al., 2002). This finding confirms that alterations in the BDNF-TrkB pathway led to a reduction in neurogenesis, which indicated that BDNF-TrkB signaling plays a key role in the pathophysiology of depression and mechanism of antidepressant treatment (Shirayama et al., 2002). Hayley et al. (2015) reported that the level of BDNF in the brains of depressed patients with suicidal tendency was lower than that of patients without suicidal tendency, suggesting that the level of BDNF may be related to the severity of depression. More severe the depressive symptoms were associated with lower levels of BDNF in the brain. An imbalance of production and release of 5-HT is the main cause of major depressive disorder (MDD). Moreover, BDNF can promote the function and growth of 5-HT neurons in the brain (Mamounas et al., 2000), indicating that the level of BDNF in MDD is critical. Some scholars believe that levels of serum BDNF can represent the levels of BDNF in the brain to a certain extent, which may be used as an indicator to evaluate the severity of depressive symptoms (Peng et al., 2018). However, Hellweg et al. (2008) tested BDNF levels in depressed patients with different antidepressants and found that changes in serum concentrations caused by antidepressants depended on the drugs rather than the general pathophysiological response of the subjects after antidepressant administration. Many studies have shown that BDNF is closely related to depression, however, the relationship between BDNF levels and the severity, remission, and recurrence of depression; the effect and enhanced expression of antidepressants on BDNF, the maintenance of long-term antidepressant treatment and the examination of ideal BDNF levels remain to be explored.
Brain-Derived Neurotrophic Factor and Anxiety
Anxiety, also known as anxiety neurosis, exists as two clinically common forms: generalized anxiety and panic disorder. Generalized anxiety is characterized by continuous nervousness, excessive alertness, and autonomic nervous dysfunction. Panic disorder is characterized by recurrent autonomic symptoms (e.g., palpitation, sweating, and tremors), as well as an irrational fear of unfortunate consequences. Epidemiological studies have revealed that approximately 30% anxiety disorder cases are hereditary. Anxiety disorder is an organic disease with physiological and biochemical abnormalities in the brain, particularly those associated with changes in the amygdala, hippocampus, hypothalamus, and frontal cortex (Mah et al., 2016). The BDNF Val66Met polymorphism is the most common structural variation of BDNF, in which a valine (Val) at codon 66 is replaced by methionine (Met). The Met allele affects the secretion and transportation of BDNF within cells (Egan et al., 2003). As a result, changes in the spatial conformation of the synaptic cleft and the growth morphology of neurons lead to the degeneration of the neural structure and an impairment in synaptic plasticity. In a study of BDNF polymorphisms, it was found that adults with the Met allele had poorer memory and smaller hippocampal volume compared with that of normal adults (Lim et al., 2017). Since BDNF is concentrated in the hippocampus and amygdala, the BDNF mononucleotide polymorphism, Val66Met, has been implicated in the pathogenesis of anxiety disorder (Mühlberger et al., 2014; Meier and Deckert, 2019). Chen et al. (2006) found that compared with normal mice, BDNFMet mice had impaired memory and reduced hippocampal volume due to changes in the dendritic shape of the dentate gyrus, confirming that decreased BDNF signaling can lead to damage to hippocampal morphology and function, and these changes may increase anxiety-related behavior. Therefore, the Val66Met polymorphism of the BDNF gene could affect both hippocampal structure and function (Bueller et al., 2006). Additionally, external factors (e.g., social stress) can lead to anxiety, as the levels of brain BDNF have been found to decrease under stressful environment (Berry et al., 2012). Although there is no definitive explanation for the relationship between these genes and anxiety, the discovery of BDNF Val66Met continues to hold promise for the development of effective drugs that target these genes for the treatment of anxiety disorders.
Brain-Derived Neurotrophic Factor and Neurodegenerative Diseases
Brain-Derived Neurotrophic Factor and Alzheimer’s Disease
Alzheimer’s disease is the most common type of dementia, characterized by progressive cognitive decline (e.g., memory, language, and behavior), resulting in the loss of the ability to use tools of daily life and carry out basic activities (No authors listed, 2020). The pathological characteristics of AD consist of the senile plaques formed by the accumulation of β-amyloid peptide (Aβ) outside of brain neurons, neurofibrillary tangles produced by the hyperphosphorylation of tau protein in the neurons and loss of neurons (Soria Lopez et al., 2019). A study has shown that Aβ decreases the level of BDNF primarily by reducing phosphorylated cAMP response element binding protein (CREB) (Garzon and Fahnestock, 2007). Furthermore, the hyperphosphorylation of tau protein downregulates the BDNF transcription process both in vivo and in vitro, while tau protein knockdown partially rescues the Aβ induced BDNF downregulation (Rosa et al., 2016). Injection of BDNF into the hippocampus of AD model mice can rescue the deficits of hippocampal synaptic long term potentiation (Nagahara et al., 2009). There are pathological changes in the AD cerebellum including granule cell dendrites and dendritic spine loss and Purkinje cell loss (Larner, 1997), and it has been found that exogenous BDNF can improve the pathology in the cerebellum associated with AD (Carter et al., 2002). The above confirmed that BDNF expression is closely related to AD pathological process. The study by Buchman et al. (2016) evaluated the cognition of 535 elderly participants year by year, and the level of BDNF in their brain after death was measured. The rate of cognitive decline was negatively correlated with the level of BDNF expression in the brain. Further analysis showed that the relationship between AD pathology and the rate of cognitive decline differed due to the level of BDNF expression. Rex et al. (2006) considered that BDNF and its receptor (TrkB) were impaired with age in AD patients. It was also suggested that β-amyloid protein was detrimental to the production and signaling of BDNF. In addition, exercise helps to increase the levels of BDNF, which can improve AD performance to some extent (Laurin et al., 2001; Etnier et al., 2016; Choi et al., 2018). Taken together, BDNF can reduce the toxicity of Aβ to neurons and enhance learning and memory capability. Thus, the application of BDNF may potentially be used to prevent and treat AD.
Brain-Derived Neurotrophic Factor and Parkinson’s Disease
Parkinson’s disease is the second most common neurodegenerative disease after AD. The primary pathological change is the degeneration and death of dopamine (DA) neurons in the substantia nigra of the midbrain, which leads to a significant decrease in the level of DA in the striatum. The cause of DA neuron death is not fully understood, and the intracellular accumulation of a-synuclein is thought to be mainly responsible for the loss of the neurons. The main histopathological features of PD are the presence of dystrophic neurons and Lewy bodies in the surviving neurons (Wakabayashi et al., 2007). Clinically, PD manifests primarily as static tremor, bradykinesia, myotonia, and postural gait disorder. Moreover, the prevalence rate increases with age (Frisardi et al., 2016). BDNF can promote the survival and differentiation of dopaminergic neurons and inhibit the degeneration of dopaminergic neurons caused by neurotoxicity, thereby improving PD (Kim et al., 2021). The study by Huang et al. (2019) found that the decrease in the level of peripheral BDNF/TrkB in PD patients was directly related to the degeneration of dopaminergic neurons. In an experiment involving monkeys, PD symptoms were significantly alleviated in the BDNF treatment group (Tsukahara et al., 1995). Moreover, BDNF Val66Met has also been indicated to be related to PD cognitive impairment (Altmann et al., 2016). Moreover, overexpression of a-synuclein downregulates the transcription and transport of BDNF in the neurons (Yuan et al., 2010). Levivier et al. (1995) used genetic engineering technology to study a rat model of PD and found that the transplantation of BDNF-producing fibroblasts into the brain could prevent the degeneration of dopamine neurons in the brain of adult rats. This finding provided direct evidence for the treatment of PD neurodegeneration by gene therapy and neurotrophic factors (e.g., BDNF).
Brain-Derived Neurotrophic Factor and Huntington’s Disease
Huntington’s disease, also known as chronic progressive dance disease, is a slow onset hereditary neurodegenerative disease, with an incidence of approximately 1 in 10,000 people. HD patients tend to appear between 30 and 40 years old and primarily manifest as involuntary dance-like body movements, cognitive dysfunction, and mental disorders. HD is an autosomal dominant neurodegenerative disease, which is primarily caused by a mutation in the Huntingtin (HTT) gene on chromosome 4, producing the mutated Huntingtin protein, which is widely expressed in neurons (No authors listed, 1993). Studies have shown that HTT is an antiapoptotic protein in striatum cells, which can prevent caspase activation. In the cells of the CNS, HTT expression can protect from lethal stimulation under normal condition (Bhide et al., 1996). However, this intracellular protein metabolic disorder causes an over-aggregation to form large molecular clusters, which will affect the normal function of nerve cells and lead to the occurrence of HD. HTT over-expression is particularly observed in damaged neurons in the striatum (Reiner et al., 1988). Yu et al. (2018) showed that the toxicity generated during the aggregation of small HTT fragments could lead to neuronal death, and BDNF expression in the striatum was also decreased. This was because BDNF was produced by the cerebral cortex and subsequently transported to the striatum. A mutation in HTT decreases the expression of BDNF, which is also associated with a reduction in the level of BDNF in the striatum (Zuccato et al., 2001). Furthermore, the activity of the BDNF/TrkB pathway was reduced by HTT and in turn aggravates striatum neuron injury, in human cerebral cortex samples, examined at postmortem, confirmed that the level of cerebral cortex BDNF in HD patients was found to be significantly lower (Zuccato et al., 2008). These findings suggest that BDNF signaling is involved in the occurrence and development of HD, and the regulation of BDNF signaling may have the potential to treat HD.
Brain-Derived Neurotrophic Factor and the Cerebellar Ataxia
The cerebellum is responsible for coordination and fine regulation of movement, and its dysfunction can cause motor ataxia. BDNF is highly expressed in cerebellar granule cells and Purkinje cells, and BDNF deficiency can result in abnormality of morphology of the cells, especially their synapses (Carter et al., 2002; Salomova et al., 2020). In BDNF knockout mice, migration of cerebellar granule cells is impaired, and is relieved after administration of exogenous BDNF (Borghesani et al., 2002). Stg mouse is a mutant mouse model with cerebellar ataxia, BDNF is significantly reduced in the cerebellum other than other brain regions of the mouse (Qiao et al., 1996). Meng et al. (2007) produced stg-BDNF double mutant mice by crossing stg mice with BDNF over-expression transgenic mice. Compared with stg mice, the coordination of movements of stg-BDNF mice was significantly improved. Moreover, a study showed that after human limbs had been trained, the volume of cerebellar gray matter and the level of BDNF in the saliva of the subjects increased, and a positive correlation was found between the two parameters (Ben-Soussan et al., 2015). All of these indicate that BDNF provides important support for the morphology and function of cerebellum.
Clinical Application of Brain-Derived Neurotrophic Factor
Although a large number of preclinical studies have provided evidence regarding the therapeutic potential of BDNF, it has been difficult to translate the work into clinical practice. The study by Benraiss et al. (2013) used an adeno-associated virus (AAV) vector to express BDNF in striatum neurons, and proved that AAV delivered BDNF-induced neurogenesis and promoted longer neuron survival in a mouse model of HD. Despite this success, the clinical use of AAV remains difficult due to the immunogenicity and biological distribution of the virus in the host (Hudry and Vandenberghe, 2019). Furthermore, BDNF has an extremely short half-life, which severely limits the effectiveness of recombinant proteins. The use of recombinant proteins is riddled with problems, including protein degradation, the immune response, and inability to cross the blood-brain barrier in large quantities. Currently, a variety of drug delivery methods have been explored in preclinical studies, including: (1) intracerebral perfusion or intracerebral injection; (2) route of viral gene therapy administration; (3) liposome encapsulated drugs; and (4) monoclonal antibody conjugated drugs for intravenous administration. However, each method has its drawbacks, and there is currently a lack of safe and effective delivery methods in clinical practice.
Outlook and Summary
Multiple studies have demonstrated that BDNF has therapeutic potential for promoting axonal regeneration, maintaining synaptic strength, preventing neuron loss in several neurodegenerative disease models, and inducing neuronal redifferentiation in acute CNS injury. However, the current understanding of the role and mechanism of BDNF in CNS diseases is not intensive, and many aspects must be explored further. Some examples of such questions include: what is the mechanism by which BDNF mediates structural and functional plasticity in the CNS under physiological circumstances? What is the specific mechanism of BDNF production, neuroprotective effect, and regulation of glial cell function in acute brain injury (e.g., stroke or brain trauma) and its recovery period? What are the changes that occur in the production and protective effects of BDNF in the pathological process of chronic ischemic brain injury (e.g., chronic hypoperfusion) and neurodegenerative diseases (e.g., AD and PD)? On the other hand, the establishment and improvement of the determination of the level of BDNF in the brain of patients in clinical application; the definition of clinical significance of peripheral and central BDNF levels in CNS disease pathogenesis; the methods by which exogenous BDNF efficiently permeates the blood-brain barrier and accurately reaches the lesion location remain to be further explored. Taken together, these findings indicate that BDNF and its downstream signaling play a key role under normal conditions, as well as in the pathological states of many neurological diseases. However, its detailed mechanism and application remains to be clarified and established in future studies.
This article discusses the role of BDNF in the pathogenesis of CNS diseases, providing a new theoretical reference for the exploration of the pathogenesis of CNS diseases, as well as their clinical diagnosis and treatment. Future in-depth studies on BDNF will be of great significance for determining the diagnosis and treatment of the neurological diseases mentioned in this article. It is believed that with further development of clinical application methods, BDNF will become an effective means of treating these diseases in the future.
Author Contributions
S-HD wrote the manuscript. YC wrote part of the manuscript. S-MH and BZ provided the critical comments and revised the manuscript. All authors approved the final draft and agreed to be accountable for all aspects of the work.
Funding
This work was supported by National Natural Science Foundation of China (Nos. 81873108 and 81603321), University Nursing Program for Young Scholars with Creative Talents in Heilongjiang Province (No. UNPYSCT-2017216), Excellent Creative Talents Support Program of Heilongjiang University of Chinese Medicine (No. 2018RCQ08), and Research Foundation of Heilongjiang University of Chinese Medicine (No. 2019BJP02).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Altmann, V., Schumacher-Schuh, A. F., Rieck, M., Callegari-Jacques, S. M., Rieder, C. R., and Hutz, M. H. (2016). Val66Met BDNF polymorphism is associated with Parkinson’s disease cognitive impairment. Neurosci. Lett. 615, 88–91.
Andero, R., Choi, D. C., and Ressler, K. J. (2014). BDNF-TrkB receptor regulation of distributed adult neural plasticity, memory formation, and psychiatric disorders. Prog. Mol. Biol. Transl. Sci. 122, 169–192. doi: 10.1016/B978-0-12-420170-5.00006-4
Barde, Y. A., Edgar, D., and Thoenen, H. (1982). Purification of a new neurotrophic factor from mammalian brain. EMBO J. 1, 549–553. doi: 10.1002/j.1460-2075.1982.tb01207.x
Benraiss, A., Toner, M. J., Xu, Q., Bruel-Jungerman, E., Rogers, E. H., and Wang, F. (2013). Sustained mobilization of endogenous neural progenitors delays disease progression in a transgenic model of Huntington’s disease. Cell Stem Cell 12, 787–799. doi: 10.1016/j.stem.2013.04.014
Ben-Soussan, T. D., Piervincenzi, C., Venditti, S., Verdone, L., Caserta, M., and Carducci, F. (2015). Increased cerebellar volume and BDNF level following quadrato motor training. Synapse 69, 1–6. doi: 10.1002/syn.21787
Berry, A., Bellisario, V., Capoccia, S., Tirassa, P., Calza, A., and Alleva, E. (2012). Social deprivation stress is a triggering factor for the emergence of anxiety- and depression-like behaviours and leads to reduced brain BDNF levels in C57BL/6J mice. Psychoneuroendocrinology 37, 762–772. doi: 10.1016/j.psyneuen.2011.09.007
Bhide, P. G., Day, M., Sapp, E., Schwarz, C., Sheth, A., and Kim, J. (1996). Expression of normal and mutant huntingtin in the developing brain. J. Neurosci. 16, 5523–5535. doi: 10.1523/JNEUROSCI.16-17-05523.1996
Borghesani, P. R., Peyrin, J. M., Klein, R., Rubin, J., Carter, A. R., and Schwartz, P. M. (2002). BDNF stimulates migration of cerebellar granule cells. Development 129, 1435–1442. doi: 10.1242/dev.129.6.1435
Brito, V., Puigdellívol, M., Giralt, A., del Toro, D., Alberch, J., and Ginés, S. (2013). Imbalance of p75(NTR)/TrkB protein expression in Huntington’s disease: implication for neuroprotective therapies. Cell Death Dis. 4:e595. doi: 10.1038/cddis.2013.116
Buchman, A. S., Yu, L., Boyle, P. A., Schneider, J. A., De Jager, P. L., and Bennett, D. A. (2016). Higher brain BDNF gene expression is associated with slower cognitive decline in older adults. Neurology 86, 735–741. doi: 10.1212/WNL.0000000000002387
Bueller, J. A., Aftab, M., Sen, S., Gomez-Hassan, D., Burmeister, M., and Zubieta, J. K. (2006). BDNF Val66Met allele is associated with reduced hippocampal volume in healthy subjects. Biol. Psychiatry 59, 812–815. doi: 10.1016/j.biopsych.2005.09.022
Carter, A. R., Chen, C., Schwartz, P. M., and Segal, R. A. (2002). Brain-derived neurotrophic factor modulates cerebellar plasticity and synaptic ultrastructure. J. Neurosci. 22, 1316–1327. doi: 10.1523/JNEUROSCI.22-04-01316.2002
Chakravarthy, B., Ménard, M., Ito, S., Gaudet, C., Dal Prà, I., and Armato, U. (2012). Hippocampal membrane-associated p75NTR levels are increased in Alzheimer’s disease. J. Alzheimers Dis. 30, 675–684. doi: 10.3233/JAD-2012-120115
Chen, H. H., Zhang, N., Li, W. Y., Fang, M. R., Zhang, H., and Fang, Y. S. (2015). Overexpression of brain-derived neurotrophic factor in the hippocampus protects against post-stroke depression. Neural Regen. Res. 10, 1427–1432. doi: 10.4103/1673-5374.165510
Chen, Z. Y., Jing, D., Bath, K. G., Ieraci, A., Khan, T., and Siao, C. J. (2006). Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science 314, 140–143. doi: 10.1126/science.1129663
Choi, S. H., Bylykbashi, E., Chatila, Z. K., Lee, S. W., Pulli, B., and Clemenson, G. D. (2018). Combined adult neurogenesis and BDNF mimic exercise effects on cognition in an Alzheimer’s mouse model. Science 361:6406. doi: 10.1126/science.aan8821
Clarke, L. E., and Barres, B. A. (2013). Emerging roles of astrocytes in neural circuit development. Nat. Rev. Neurosci. 14, 311–321. doi: 10.1038/nrn3484
Egan, M. F., Kojima, M., Callicott, J. H., Goldberg, T. E., Kolachana, B. S., and Bertolino, A. (2003). The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell 112, 257–269.
Etnier, J. L., Wideman, L., Labban, J. D., Piepmeier, A. T., Pendleton, D. M., and Dvorak, K. K. (2016). The Effects of Acute Exercise on Memory and Brain-Derived Neurotrophic Factor (BDNF). J. Sport Exerc. Psychol. 38, 331–340. doi: 10.1123/jsep.2015-0335
Frisardi, V., Santamato, A., and Cheeran, B. (2016). Parkinson’s Disease: New Insights into Pathophysiology and Rehabilitative Approaches. Parkinsons Dis. 2016:3121727. doi: 10.1155/2016/3121727
Garzon, D. J., and Fahnestock, M. (2007). Oligomeric amyloid decreases basal levels of brain-derived neurotrophic factor (BDNF) mRNA via specific downregulation of BDNF transcripts IV and V in differentiated human neuroblastoma cells. J. Neurosci. 27, 2628–2635. doi: 10.1523/JNEUROSCI.5053-06.2007
Hayley, S., Du, L., Litteljohn, D., Palkovits, M., Faludi, G., and Merali, Z. (2015). Gender and brain regions specific differences in brain derived neurotrophic factor protein levels of depressed individuals who died through suicide. Neurosci. Lett. 600, 12–16. doi: 10.1016/j.neulet.2015.05.052
Hellweg, R., Ziegenhorn, A., Heuser, I., and Deuschle, M. (2008). Serum concentrations of nerve growth factor and brain-derived neurotrophic factor in depressed patients before and after antidepressant treatment. Pharmacopsychiatry 41, 66–71. doi: 10.1055/s-2007-1004594
Holt, L. M., Hernandez, R. D., Pacheco, N. L., Torres Ceja, B., Hossain, M., and Olsen, M. L. (2019). Astrocyte morphogenesis is dependent on BDNF signaling via astrocytic TrkB.T1. eLife 8:e44667. doi: 10.7554/eLife.44667
Hsu, C. C., Kuo, T. W., Liu, W. P., Chang, C. P., and Lin, H. J. (2020). Calycosin Preserves BDNF/TrkB Signaling and Reduces Post-Stroke Neurological Injury after Cerebral Ischemia by Reducing Accumulation of Hypertrophic and TNF-α-Containing Microglia in Rats. J. Neuroimmune Pharmacol. 15, 326–339. doi: 10.1007/s11481-019-09903-9
Huang, Y., Huang, C., and Yun, W. (2019). Peripheral BDNF/TrkB protein expression is decreased in Parkinson’s disease but not in Essential tremor. J. Clin. Neurosci. 63, 176–181. doi: 10.1016/j.jocn.2019.01.017
Hudry, E., and Vandenberghe, L. H. (2019). Therapeutic AAV Gene Transfer to the Nervous System: A Clinical Reality. Neuron 101, 839–862.
Ibáñez, C. F., and Simi, A. (2012). p75 neurotrophin receptor signaling in nervous system injury and degeneration: paradox and opportunity. Trends Neurosci. 35, 431–440. doi: 10.1016/j.tins.2012.03.007
Kim, H. I., Lee, S., Lim, J., Chung, S., Koo, T. S., and Ji, Y. G. (2021). ERRγ ligand HPB2 upregulates BDNF-TrkB and enhances dopaminergic neuronal phenotype. Pharmacol. Res. 165:105423. doi: 10.1016/j.phrs.2021.105423
Kokaia, Z., Andsberg, G., Yan, Q., and Lindvall, O. (1998). Rapid alterations of BDNF protein levels in the rat brain after focal ischemia: evidence for increased synthesis and anterograde axonal transport. Exp. Neurol. 154, 289–301. doi: 10.1006/exnr.1998.6888
Kowiański, P., Lietzau, G., Czuba, E., Waśkow, M., Steliga, A., and Moryś, J. (2018). BDNF: A Key Factor with Multipotent Impact on Brain Signaling and Synaptic Plasticity. Cell. Mol. Neurobiol. 38, 579–593. doi: 10.1007/s10571-017-0510-4
Kurozumi, K., Nakamura, K., Tamiya, T., Kawano, Y., Kobune, M., and Hirai, S. (2004). BDNF gene-modified mesenchymal stem cells promote functional recovery and reduce infarct size in the rat middle cerebral artery occlusion model. Mol. Ther. 9, 189–197. doi: 10.1016/j.ymthe.2003.10.012
Larner, A. J. (1997). The cerebellum in Alzheimer’s disease: evaluating its role in cognitive decline. Brain 8, 203–209. doi: 10.1093/brain/awx194
Laurin, D., Verreault, R., Lindsay, J., MacPherson, K., and Rockwood, K. (2001). Physical activity and risk of cognitive impairment and dementia in elderly persons. Arch. Neurol. 58, 498–504. doi: 10.1001/archneur.58.3.498
Levivier, M., Przedborski, S., Bencsics, C., and Kang, U. J. (1995). Intrastriatal implantation of fibroblasts genetically engineered to produce brain-derived neurotrophic factor prevents degeneration of dopaminergic neurons in a rat model of Parkinson’s disease. J. Neurosci. 15, 7810–7820. doi: 10.1523/JNEUROSCI.15-12-07810.1995
Levy, M. J. F., Boulle, F., Steinbusch, H. W., van den Hove, D. L. A., Kenis, G., and Lanfumey, L. (2018). Neurotrophic factors and neuroplasticity pathways in the pathophysiology and treatment of depression. Psychopharmacology 235, 2195–2220. doi: 10.1007/s00213-018-4950-4
Li, J., Chen, J., Ma, N., Yan, D., Wang, Y., and Zhao, X. (2019). Effects of corticosterone on the expression of mature brain-derived neurotrophic factor (mBDNF) and proBDNF in the hippocampal dentate gyrus. Behav. Brain Res. 365, 150–156. doi: 10.1016/j.bbr.2019.03.010
Li, Y., Luikart, B. W., Birnbaum, S., Chen, J., Kwon, C. H., and Kernie, S. G. (2008). TrkB regulates hippocampal neurogenesis and governs sensitivity to antidepressive treatment. Neuron 59, 399–412. doi: 10.1016/j.neuron.2008.06.023
Lim, Y. Y., Rainey-Smith, S., Lim, Y., Laws, S. M., Gupta, V., and Porter, T. (2017). BDNF Val66Met in preclinical Alzheimer’s disease is associated with short-term changes in episodic memory and hippocampal volume but not serum mBDNF. Int. Psychogeriatr. 29, 1825–1834. doi: 10.1017/S1041610217001284
Louveau, A., Nerrière-Daguin, V., Vanhove, B., Naveilhan, P., Neunlist, M., and Nicot, A. (2015). Targeting the CD80/CD86 costimulatory pathway with CTLA4-Ig directs microglia toward a repair phenotype and promotes axonal outgrowth. Glia 63, 2298–2312. doi: 10.1002/glia.22894
Mah, L., Szabuniewicz, C., and Fiocco, A. J. (2016). Can anxiety damage the brain? Curr. Opin. Psychiatry 29, 56–63.
Mamounas, L. A., Altar, C. A., Blue, M. E., Kaplan, D. R., Tessarollo, L., and Lyons, W. E. (2000). BDNF promotes the regenerative sprouting, but not survival, of injured serotonergic axons in the adult rat brain. J. Neurosci. 20, 771–782. doi: 10.1523/JNEUROSCI.20-02-00771.2000
Meeker, R. B., and Williams, K. S. (2015). The p75 neurotrophin receptor: at the crossroad of neural repair and death. Neural Regen. Res. 10, 721–725. doi: 10.4103/1673-5374.156967
Meng, H., Larson, S. K., Gao, R., and Qiao, X. (2007). BDNF transgene improves ataxic and motor behaviors in stargazer mice. Brain Res. 1160, 47–57. doi: 10.1016/j.brainres.2007.05.048
Mühlberger, A., Andreatta, M., Ewald, H., Glotzbach-Schoon, E., Tröger, C., and Baumann, C. (2014). The BDNF Val66Met polymorphism modulates the generalization of cued fear responses to a novel context. Neuropsychopharmacology 39, 1187–1195. doi: 10.1038/npp.2013.320
Nagahara, A. H., Merrill, D. A., Coppola, G., Tsukada, S., Schroeder, B. E., and Shaked, G. M. (2009). Neuroprotective effects of brain-derived neurotrophic factor in rodent and primate models of Alzheimer’s disease. Nat. Med. 15, 331–337. doi: 10.1038/nm.1912
Nagahara, A. H., and Tuszynski, M. H. (2011). Potential therapeutic uses of BDNF in neurological and psychiatric disorders. Nat. Rev. Drug Discov. 10, 209–219. doi: 10.1038/nrd3366
No authors listed (1993). A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. The Huntington’s Disease Collaborative Research Group. Cell 72, 971–983.
No authors listed (2020). 2020 Alzheimer’s disease facts and figures. Alzheimers Dement. [Epub online ahead of print]. doi: 10.1002/alz.12068
Peng, S., Li, W., Lv, L., Zhang, Z., and Zhan, X. (2018). BDNF as a biomarker in diagnosis and evaluation of treatment for schizophrenia and depression. Discov. Med. 26, 127–136.
Pruunsild, P., Kazantseva, A., Aid, T., Palm, K., and Timmusk, T. (2007). Dissecting the human BDNF locus: bidirectional transcription, complex splicing, and multiple promoters. Genomics 90, 397–406. doi: 10.1016/j.ygeno.2007.05.004
Qiao, H., An, S. C., Xu, C., and Ma, X. M. (2017). Role of proBDNF and BDNF in dendritic spine plasticity and depressive-like behaviors induced by an animal model of depression. Brain Res. 1663, 29–37. doi: 10.1016/j.brainres.2017.02.020
Qiao, X., Hefti, F., Knusel, B., and Noebels, J. L. (1996). Selective failure of brain-derived neurotrophic factor mRNA expression in the cerebellum of stargazer, a mutant mouse with ataxia. J. Neurosci. 16, 640–648. doi: 10.1523/JNEUROSCI.16-02-00640.1996
Quesseveur, G., David, D. J., Gaillard, M. C., Pla, P., Wu, M. V., and Nguyen, H. T. (2013). BDNF overexpression in mouse hippocampal astrocytes promotes local neurogenesis and elicits anxiolytic-like activities. Transl. Psychiatry 3:e253. doi: 10.1038/tp.2013.30
Reiner, A., Albin, R. L., Anderson, K. D., D’Amato, C. J., Penney, J. B., and Young, A. B. (1988). Differential loss of striatal projection neurons in Huntington disease. Proc. Natl. Acad. Sci. U.S.A. 85, 5733–5737. doi: 10.1073/pnas.85.15.5733
Rex, C. S., Lauterborn, J. C., Lin, C. Y., Kramár, E. A., Rogers, G. A., and Gall, C. M. (2006). Restoration of long-term potentiation in middle-aged hippocampus after induction of brain-derived neurotrophic factor. J. Neurophysiol. 96, 677–685. doi: 10.1152/jn.00336.2006
Rosa, E., Mahendram, S., Ke, Y. D., Ittner, L. M., Ginsberg, S. D., and Fahnestock, M. (2016). Tau downregulates BDNF expression in animal and cellular models of Alzheimer’s disease. Neurobiol. Aging 48, 135–142. doi: 10.1016/j.neurobiolaging.2016.08.020
Saarelainen, T., Hendolin, P., Lucas, G., Koponen, E., Sairanen, M., and MacDonald, E. (2003). Activation of the TrkB neurotrophin receptor is induced by antidepressant drugs and is required for antidepressant-induced behavioral effects. J. Neurosci. 23, 349–357. doi: 10.1523/JNEUROSCI.23-01-00349.2003
Salomova, M., Tichanek, F., Jelinkova, D., and Cendelin, J. (2020). Abnormalities in the cerebellar levels of trophic factors BDNF and GDNF in pcd and lurcher cerebellar mutant mice. Neurosci. Lett. 725:134870. doi: 10.1016/j.neulet.2020.134870
Schäbitz, W. R., Steigleder, T., Cooper-Kuhn, C. M., Schwab, S., Sommer, C., and Schneider, A. (2007). Intravenous brain-derived neurotrophic factor enhances poststroke sensorimotor recovery and stimulates neurogenesis. Stroke 38, 2165–2172. doi: 10.1161/STROKEAHA.106.477331
Shirayama, Y., Chen, A. C., Nakagawa, S., Russell, D. S., and Duman, R. S. (2002). Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J. Neurosci. 22, 3251–3261. doi: 10.1523/JNEUROSCI.22-08-03251.2002
Siuciak, J. A., Lewis, D. R., Wiegand, S. J., and Lindsay, R. M. (1997). Antidepressant-like effect of brain-derived neurotrophic factor (BDNF). Pharmacol. Biochem. Behav. 56, 131–137.
Song, M., Martinowich, K., and Lee, F. S. (2017). BDNF at the synapse: why location matters. Mol. Psychiatry 22, 1370–1375. doi: 10.1038/mp.2017.144
Soria Lopez, J. A., González, H. M., and Léger, G. C. (2019). Alzheimer’s disease. Handb. Clin. Neurol. 167, 231–255.
Tsukahara, T., Takeda, M., Shimohama, S., Ohara, O., and Hashimoto, N. (1995). Effects of brain-derived neurotrophic factor on 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced parkinsonism in monkeys. Neurosurgery 37, 733–739. ; discussion 9-41. doi: 10.1227/00006123-199510000-00018
Wakabayashi, K., Tanji, K., Mori, F., and Takahashi, H. (2007). The Lewy body in Parkinson’s disease: molecules implicated in the formation and degradation of alpha-synuclein aggregates. Neuropathology 27, 494–506. doi: 10.1111/j.1440-1789.2007.00803.x
Woo, N. H., Teng, H. K., Siao, C. J., Chiaruttini, C., Pang, P. T., and Milner, T. A. (2005). Activation of p75NTR by proBDNF facilitates hippocampal long-term depression. Nat. Neurosci. 8, 1069–1077. doi: 10.1038/nn1510
Yang, B., Wang, L., Nie, Y., Wei, W., and Xiong, W. (2021). proBDNF expression induces apoptosis and inhibits synaptic regeneration by regulating the RhoA-JNK pathway in an in vitro post-stroke depression model. Transl. Psychiatry 11:578. doi: 10.1038/s41398-021-01667-2
Yu, C., Li, C. H., Chen, S., Yoo, H., Qin, X., and Park, H. (2018). Decreased BDNF Release in Cortical Neurons of a Knock-in Mouse Model of Huntington’s Disease. Sci. Rep. 8:16976. doi: 10.1038/s41598-018-34883-w
Yuan, Y., Sun, J., Zhao, M., Hu, J., Wang, X., and Du, G. (2010). Overexpression of alpha-synuclein down-regulates BDNF expression. Cell. Mol. Neurobiol. 30, 939–946. doi: 10.1007/s10571-010-9523-y
Zhao, X. P., Li, H., and Dai, R. P. (2022). Neuroimmune crosstalk through brain-derived neurotrophic factor and its precursor pro-BDNF: New insights into mood disorders. World J. Psychiatry 12, 379–392. doi: 10.5498/wjp.v12.i3.379
Zhou, L., Xiong, J., Lim, Y., Ruan, Y., Huang, C., and Zhu, Y. (2013). Upregulation of blood proBDNF and its receptors in major depression. J. Affect. Disord. 150, 776–784. doi: 10.1016/j.jad.2013.03.002
Zuccato, C., Ciammola, A., Rigamonti, D., Leavitt, B. R., Goffredo, D., and Conti, L. (2001). Loss of huntingtin-mediated BDNF gene transcription in Huntington’s disease. Science 293, 493–498. doi: 10.1126/science.1059581
Keywords: brain-derived neurotrophic factor, TrkB, stroke, depression, anxiety, neurodegenerative disease
Citation: Dou S-H, Cui Y, Huang S-M and Zhang B (2022) The Role of Brain-Derived Neurotrophic Factor Signaling in Central Nervous System Disease Pathogenesis. Front. Hum. Neurosci. 16:924155. doi: 10.3389/fnhum.2022.924155
Received: 20 April 2022; Accepted: 31 May 2022;
Published: 24 June 2022.
Edited by:
Björn H. Schott, Leibniz Institute for Neurobiology (LG), GermanyReviewed by:
Sonia Canterini, Sapienza University of Rome, ItalyRainer Hellweg, Charité – University Medicine Berlin, Germany
Copyright © 2022 Dou, Cui, Huang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bo Zhang, hljzyyzb@163.com
 Shu-Hui Dou
Shu-Hui Dou Yu Cui
Yu Cui Shu-Ming Huang
Shu-Ming Huang Bo Zhang
Bo Zhang