- Health Psychology, KU Leuven, Leuven, Belgium
Background: In asthma and allergic rhinitis, beliefs about what triggers allergic reactions often do not match objective allergy tests. This may be due to insensitivity for expectancy violations as a result of holding trigger beliefs based on conceptual relationships among triggers. In this laboratory experiment, we aimed to investigate how pre-existing beliefs and conceptual relationships among triggers interact with actual experience when learning differential symptom expectations.
Methods: Healthy participants (N = 48) received information that allergic reactions were a result of specific sensitivities versus general allergic vulnerability. Next, they performed a trigger learning task using a differential conditioning paradigm: brief inhalation of CO2 enriched air was used to induce symptoms, while participants were led to believe that the symptoms came about as a result of inhaled allergens (conditioned stimuli, CS’s; CS+ followed by symptoms, CS- not followed by symptoms). CS+ and CS- stimuli either shared (e.g., birds-mammals) or did not share (e.g. birds-fungi) category membership. During Acquisition, participants reported symptom expectancy and symptom intensity for all triggers. During a Test 1 day later, participants rated symptom expectancies for old CS+/CS- triggers, for novel triggers within categories, and for exemplars of novel trigger categories. Data were analyzed using multilevel models.
Findings: Only a subgroup of participants (n = 22) showed differences between CO2 and room air symptoms. In this group of responders, analysis of symptom expectancies during acquisition did not result in significant differential symptom CS+/CS- acquisition. A retention test 1 day later showed differential CS+/CS- symptom expectancies: When CS categories did not share category membership, specific sensitivity beliefs improved retention of CS+/CS- differentiation. However, when CS categories shared category membership, general vulnerability beliefs improved retention of CS+/CS- differentiation. Furthermore, participants showed some selectivity in generalization of symptom expectancies to novel categories, as symptom expectancies did not generalize to novel categories that were unrelated to CS+ or CS- categories. Generalization to novel categories was not affected by information about general vulnerability or specific sensitivities.
Discussion: Pre-existing vulnerability beliefs and conceptual relationships between trigger categories influence differential symptom expectancies to allergic triggers.
Introduction
Asthma and allergic rhinitis are chronic conditions that are characterized by an allergic or hyperreactive response of the airways to a variety of triggers (Bousquet et al., 2012; Global Initiative for Asthma (GINA), 2016). Because treatment for these conditions is currently not available, management strategies are suggested to reduce the manifestation of symptoms and increase clinical control (Global Initiative for Asthma (GINA), 2016). These management strategies are multifaceted, and include pharmacological strategies (often a combination of preventer and reliever medication) as well as behavioral strategies of trigger identification and subsequent avoidance as a way to obtain control over symptoms (Global Initiative for Asthma (GINA), 2016). However, despite these treatment options day-to-day control over symptoms is often poor (Rabe et al., 2004; Peters et al., 2007).
One reason for the lack of day-to-day symptom control may be the difficulties that arise when implementing trigger identification and behavioral avoidance strategies (Janssens and Ritz, 2013). These latter strategies rely on the perception of spatio-temporal contingencies between the presence of triggers and subsequent emergence of asthmatic or allergic symptoms in order to allow prediction of symptoms and accurate avoidance of triggers. In other words, based on medical information and personal experiences, patients construct trigger beliefs to guide their (future) behavior. Interestingly, trigger beliefs often do not match with the results of a structured trigger evaluation procedure, with both false positives and false negatives being observed (Li et al., 2000; Smith et al., 2009). Furthermore, in day-to-day asthma management, individuals with asthma often report being uncertain about their personal triggers and trigger avoidance strategies (Caress et al., 2002; Trollvik and Severinsson, 2004). In addition, individuals show a marked variation in the type and number of asthma triggers they identify, with a higher number of self-identified asthma triggers being associated with worse asthma outcomes, even when controlling for other measures of asthma severity (Ritz et al., 2006, 2016; Janssens and Harver, 2015). Taken together, these findings suggest difficulties and inaccuracies in the process of asthma trigger identification or the detection of trigger-symptom contingencies. Moreover, literature on symptom perception suggests that these beliefs about trigger-symptom contingencies may in turn bias perception of respiratory symptoms (Janssens et al., 2009; von Leupoldt and Dahme, 2012), which may lead to even more difficulties in trigger identification.
Previously, we have highlighted similarities between asthma trigger learning and other contingency learning tasks that occur in a motivational context, such as the identification of danger and safety that occurs within the context of fear learning (Janssens and Ritz, 2013; Janssens et al., 2015). Building upon these similarities, we have explored generalization of symptom-trigger contingencies as a potential mechanism of the observed inaccuracies in asthma trigger identification. Similar to conceptualization of generalization in the context of anxiety and fear, generalization of trigger beliefs may serve an adaptive purpose in that it helps to transfer knowledge that is gained from experience to similar instances which have not (yet) been experienced, therefore limiting the risk of adverse symptom outcomes. However, generalization may also be considered excessive or maladaptive when innocuous stimuli are treated as threatening, especially if the associated symptoms and behavioral responses interfere with day to day functioning or quality of life (Dunsmoor et al., 2009; van Meurs et al., 2014). An illustrative example in the field of allergy is the avoidance of tree nuts by individuals that show a sensitivity to peanut allergens. This avoidance seems sensible, based on considerable similarities between peanuts and tree nuts. However, a recent review of the available evidence for this strategy shows that avoidance of all tree nuts in individuals with peanut allergy may be overly precautious (Brough et al., 2015).
So far, in associative learning research, most research on generalization has studied perceptual similarities as a basis for generalization. However, recent research has also explored the role of higher order cognitions such as category membership and stimulus typicality as a basis for generalization, showing that participants can use their pre-existing knowledge about categories as a basis for fear generalization (Dunsmoor and Murphy, 2015; Dymond et al., 2015). Based on these developments in fear generalization research, we previously have adapted an associative learning or conditioning paradigm focusing on category based fear learning (Dunsmoor et al., 2012) into a lab method to investigate category-based respiratory trigger learning. Briefly, this method consists of the presentation of pictures, which are unique exemplars of two different allergen categories (e.g., mammals and flowers). Exemplars of one category (conditioned stimuli, CS+) predict onset of respiratory symptoms, whereas exemplars of the other category (CS-) are never followed by symptoms. Using this method, we observed generalization of trigger beliefs to novel category exemplars, as well as to exemplars of categories that were similar of the original trigger categories, providing a proof of concept that trigger beliefs are shaped by pre-existing conceptual knowledge (Janssens et al., 2015). Moreover, an important finding of this study was that generalization of symptom expectancies to novel CS+ exemplars was increased if participants had experienced CS+ and CS- categories that were more similar (e.g., mammals and birds), compared to categories that were more different (e.g., mammals and molds). We interpreted this finding as an effect of discrimination learning on the inferred relevance of category features as basis for generalization, which is in line with other studies that have showed an impact of either inferred or instructed feature relevance on feature based fear generalization, and support feature-extraction or rule-based accounts of generalization (Vervliet et al., 2010; Vervliet and Geens, 2014; Ahmed and Lovibond, 2015a,b).
The role of category identification and feature extraction in the generalization of cue-outcome contingencies prompts investigation into the potential role of other complex cognitive mechanisms in changing the course of generalization. More specifically, it may provide opportunities to link research on generalization with the large body of research on the role of illness-related beliefs in the context of symptom perception and disease-related behaviors (Leventhal et al., 1980; Hagger and Orbell, 2003). In asthma, research within this framework has been successful in highlighting the role of beliefs about symptom chronicity, controllability, and medication necessity and concerns, in explaining individual differences in symptom perception and medication use patterns (Horne and Weinman, 1999; Halm et al., 2006; Kaptein et al., 2010). However, research into beliefs about causality and beliefs about trigger-symptom causal chains have been limited. An exception to this is a study by McQuaid et al. (2002), who studied the cognitive complexity of causal understanding in children with asthma and their parents. In this study, participants were asked to elaborate on the question “what causes your asthma,” and “how does this trigger cause asthma symptoms.” Results of this study showed a variety of complexity of responses, ranging from phenomism (no differentiation between cause and effect) to complex psychophysiological causal models, with more complex understanding of causal chains in asthma being associated with better treatment strategies.
Building upon this study, the aim of our research was to investigate the relationship between beliefs about causality in asthma and the way individuals integrate real life experiences into models of symptom-trigger contingency. Our study provides a lab based analog for a common task in the initial treatment phase of allergy management: individuals receive information about what asthma is, and are confronted with a variety of potential triggers, that are linked to adverse outcomes (airway symptoms) in a probabilistic way. In line with our focus on causality, we chose to focus on beliefs that link asthma triggers to a general vulnerability vs. beliefs that focus on asthma triggers as very specific indicators of specific airway sensitivities, thereby mimicking different information that may be given to patients with allergic conditions by their physician or information individuals may find on the internet (Smith et al., 1998; Croft and Peterson, 2002; Huckvale et al., 2012). The actual contingencies that were presented in the task did not fully confirm or disconfirm this prior information, in that during acquisition, each potential trigger that was presented was unique. However, participants could use their knowledge of category membership and category relations to infer differences in trigger-symptom contingencies at a category level. We hypothesized that a focus on general vulnerability would hinder differentiation between triggers and non-triggers, whereas a focus on specific sensitivities would improve differentiation between triggers and non-triggers. Furthermore, in line with our previous findings on differential acquisition of trigger beliefs, we expected that the use of CS- trigger categories that were more similar to the CS+ categories would enhance differentiation between CS+ and CS- trigger beliefs.
Materials and Methods
Participants
The study was approved by the Social and Societal Ethics Committee at KU Leuven and the Ethical Review Board of Leuven University Hospitals (study ID: ML10101). Participants were 48 healthy volunteers (15 male, aged 17–38), recruited from the student population. Psychology students received course credit for participation in the experiment. The other participants received 12 euros.
Exclusion criteria were self-reported allergies, hay fever, asthma or other lung disease, heart disease, epilepsy, other severe medical or psychiatric illnesses and the presence of electronic implants. Furthermore, participants were excluded if their lung function (forced expiratory volume in 1 s) was below 80% of their predicted value.
Materials
Measures
Symptom expectancy was measured using a visual analog scale (VAS) anchored at definitely no symptoms and definite symptoms. For symptom intensity and unpleasantness, VAS were used with the anchors not at all intense/unpleasant and maximal imaginable intensity/unpleasantness.
During the online retention/generalization test, for all pictures in the trigger stimulus set, participants rated whether they had seen the picture during the lab task, or whether it was novel. Furthermore, symptom probabilities were assessed on an 11-point scale ranging from 0% (will not experience symptoms), to 100% (will definitely experience symptoms).
The Positive and Negative Affect Schedule [PANAS; (Watson et al., 1988), Dutch version (Engelen et al., 2006)] was used to assess trait positive affect and trait negative affect. The PANAS is a 20 item scale consisting of positive and negative emotion words. For each of the items, participants indicate on a 5-point scale, ranging from very little to very much, to which extend they experience each of these feelings in their daily lives.
Suffocation fear was measured using the suffocation scale of the Dutch Claustrophobia Questionnaire (CLQ; Van Diest et al., 2010). This scale consists of 14 situations that may elicit suffocation fears. Participants rate how fearful they would feel in each of the situations, on a 5-point scale ranging from not at all fearful to extremely fearful.
Stimuli
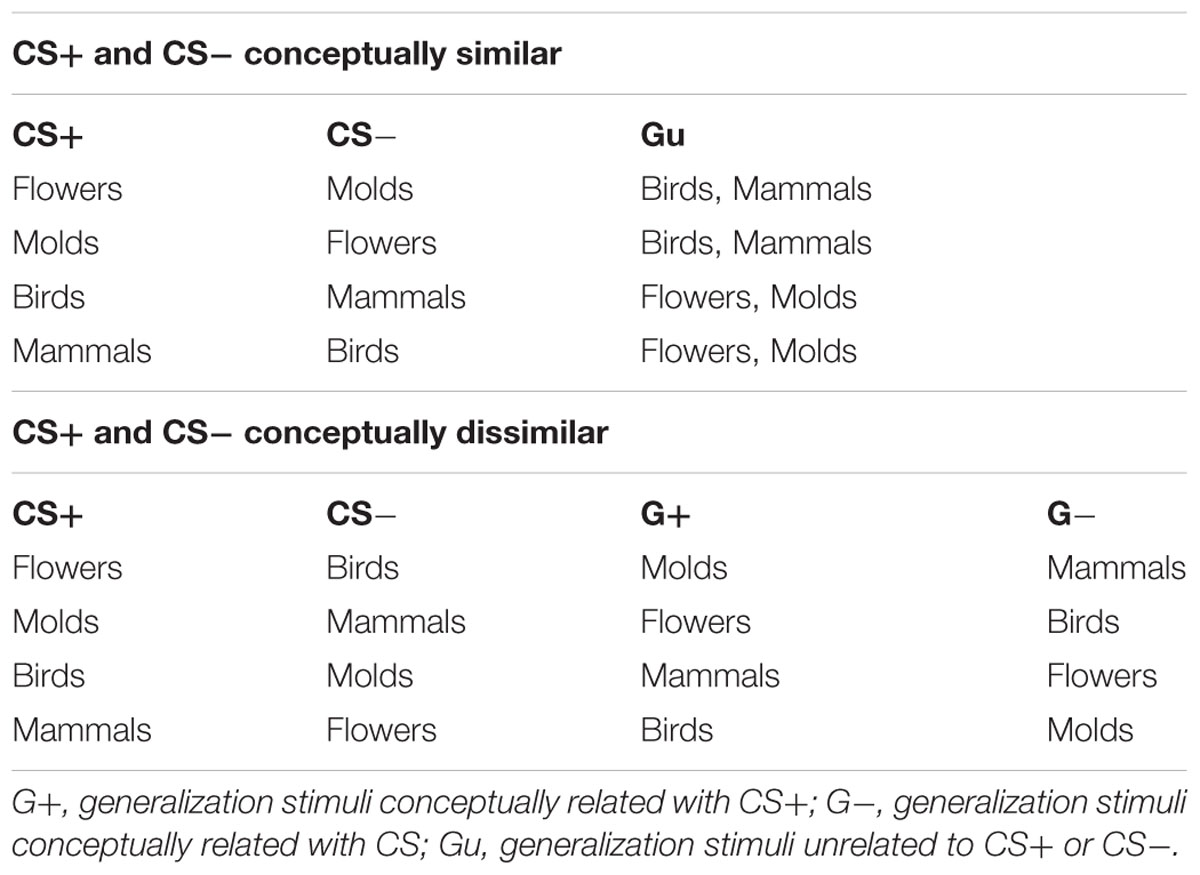
Asthma trigger stimuli consisted of four categories of potential asthma triggers: mammals, birds, flowers, and molds. This is the same stimulus set that we have used in previous research (Janssens et al., 2015). Each category consists of 20 unique pictures, and stimulus categories can be organized into two hierarchical categories: “animals” (mammals; birds) and “plants”(flowers; molds), creating the potential for constructing acquisition trigger sets with CS’s that are conceptually more/less similar. The difference in similarity between category pairs was tested and confirmed in previous research (Janssens et al., 2015). Allocation of CS+/- categories during acquisition was counterbalanced across participants, according to Table 1.
Asthma information was embedded in the informed consent form. In the general vulnerability condition, this consisted of information that asthma was an allergic condition, and that allergic responses to allergy triggers were an indication of a general vulnerability making it necessary to avoid all potential asthma triggers. The condition highlighting specific sensitivities consisted of information that asthma was the result of an allergic response to specific allergens, and that careful investigation of triggers and non-triggers was possible, so that individuals with asthma do not need to avoid a variety of potential triggers.
Apparatus
Lung function was measured using a spirometer (Jaeger Masterscope; Hoechberg, Germany) prior to the actual start of the experimental breathing trials. For the latter trials, a valve was used for switching between the regular room air and the CO2-enriched air. The CO2-enriched air consisted of a mixture of 7.5% CO2, 21% O2, and 71.5% N2 fed into a meteorological balloon. Short-term inhalation of CO2-enriched air affects respiration, increasing breathing frequency and volume, and feelings of breathlessness, mimicking aspects of asthma symptoms (De Peuter et al., 2008; Janssens et al., 2011). The participants breathed into a mask connected to the valve through an antibacterial filter. The mask was also connected to a capnograph (Nonin LifeSense, Leek, The Netherlands) and a pneumotachograph (Fleisch No. 2, fg-deutschland; Hechingen, Germany). Affect 4.0 software (Spruyt et al., 2010) was used for stimulus presentation and to record participant responses and capnograph and pneumotachograph signals.
Procedure
When participants arrived at the laboratory, they received oral and written information about the experiment. Participants were told that they would inhale a series of aerosols, each containing a mixture of air and a specific allergen, and that there was a risk of the occurrence of respiratory symptoms during these breathing trials. The information about the experiment also included our asthma information manipulation, and participants were randomly assigned to receive information focusing on general vulnerability or specific sensitivities.
After reviewing the information and exclusion criteria, participants completed informed consent. Subsequently, lung function was measured.
Subsequently, trigger acquisition trials started using a similar trial-unique acquisition procedure as in Janssens et al. (2015). The experimenter left the room and participants received 20 breathing trials. Each breathing trial followed the same pattern. First, a novel picture of a potential asthma trigger was shown, indicating to the participant that this allergen would be presented during the breathing trial (although in reality no allergens were present and symptom onset and trigger-symptom contingency was experimentally controlled). Ten pictures randomly chosen from each the CS+ and CS- trigger category was used for this purpose. After presentation of the picture, participants rated symptom expectancy using the VAS expectancy scale. Subsequently, participants were instructed to breathe through the mask, while the picture remained visible. Through the mask, the participants inhaled either regular room air either CO2-enriched air. For 6 out of the 10 CS+ trials, participants inhaled CO2-enriched air followed after the pictures. In all other trials, participants inhaled room air. After 60 s, participants could take off the mask, and rated symptom intensity and unpleasantness, using the intensity and unpleasantness VAS scales. Ratings were followed by a 2-min recovery phase, after which participants were prompted to start a new breathing trial.
One day after trigger acquisition, participants filled out an online survey. The survey consisted of the PANAS and the Suffocation scale of the CLQ, as well as recognition and symptom probability ratings of the full trigger picture set. Trigger pictures were presented in random order. After completion of the survey, participants were debriefed.
Data Reduction and Data Analysis
In order to obtain data about breathing behavior, pneumotachograph and capnograph data were processed offline using PSPHA (De Clerck et al., 2006), which resulted in breath-by-breath information of respiratory timing, respiratory volume, and fraction of end-tidal CO2 (FetCO2). Results of these analyses were further averaged for each acquisition trial.
Data analysis was carried out using SPSS 22 (IBM, Armonk, NY, United States). Symptom response to CO2 was defined as a significant within-person difference between symptom ratings after the CO2 trials, compared to room air trials, calculated using independent samples t-tests. If participants showed a p < 0.05 significant difference in symptom levels either on the symptom intensity or symptom unpleasantness ratings, they were deemed CO2 responders (n = 22). Participants not showing a significant difference were deemed non-responders (n = 26). Responders and non-responders did not differ in gender [X2(1) = 0.01, p = 0.938], age [t(46) = -0.17, p = 0.864], negative affectivity [t(46) = -0.71, p = 0.479], positive affectivity [t(46) = 0.09, p = 0.928] and fear of suffocation [t(46) = -0.96, p = 0.340], nor did they differ in assignment to information manipulation groups [X2(1) = 0.00, p = 1.000], or assignment of similar/different CS categories during acquisition [X2(1) = 0.34, p = 0.562]. Additionally, we explored difference in respiratory parameters of CO2 responders vs. non-responders. One participant was excluded from these analyses because of equipment failure. In a series of one-way repeated measures ANOVA’s, we found significant differences in the two groups for expiratory and inspiratory volume, minute ventilation, inspiratory drive, and FetCO2 and room air and the minute ventilation (Table 2).
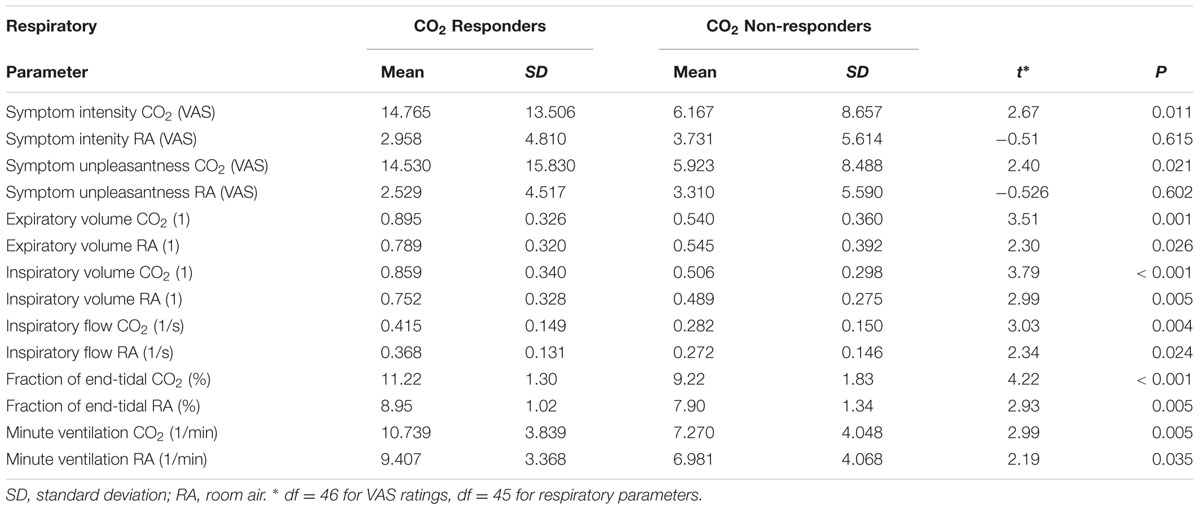
TABLE 2. Differences in Symptom Report and Respiratory Parameters between CO2 Responders and Non-responders.
Using only the responder data, acquisition, retention, and generalization of trigger beliefs was evaluated using multilevel (linear mixed models) analysis. Multilevel models were chosen because these models are less restrictive in variance-covariance assumptions for repeated measures data compared to repeated measures ANOVA, are robust to unbalanced designs, and are less restrictive in the need of having fully nested or fully crossed designs (e.g., clear separation between- and within-subject effects) compared to (repeated measures) ANOVA (Cnaan et al., 1997). Therefore, these models provide an option to deal with the peculiarities of our trigger recognition/generalization dataset (e.g., all participants having CS+ and CS- trials, but having either 20 Gu trials or 10 G+ and 10 G- trials). Models were fitted using random intercepts to account for the data being nested within participants, and were estimated using Maximum Likelihood estimation. SPSS uses Satterthwaite approximation to determine df for F-tests/t-tests. Model fit was evaluated using Akaike’s Information Criterion (AIC). We carried out additional analyses on the full set of participants, while including CO2 responder status as an additional factor. For the CO2 responders, this did not result in major changes to our findings for retention and generalization of trigger beliefs. Results of these analyses are reported as Supplementary Material.
Results
Acquisition of Trigger Beliefs
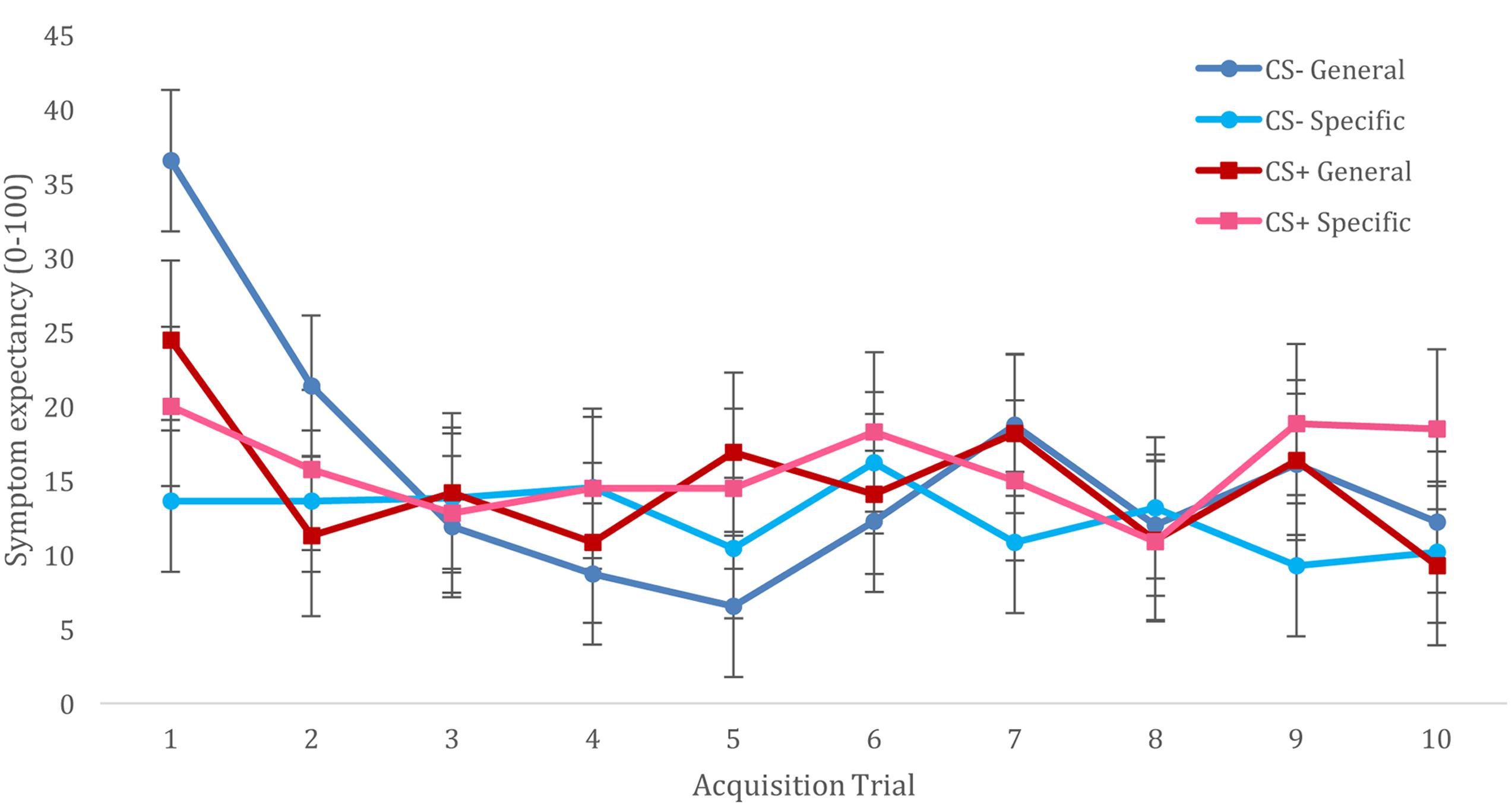
For acquisition of trigger beliefs, we constructed a multilevel model that included fixed effects of CS (CS+ vs. CS-), Trial (T1–T10), and Trigger Information (general vulnerability vs. specific sensitivities), and included all interactions between these variables. The model also included a random (individual level) effect of CS, with an unstructured variance-covariance matrix. We observed no main effects of CS type [F(1,22) = 0.260, p = 0.615] nor a CS type × trial interaction [F(9,396) = 1.190, p = 0.300]. However, this analysis resulted in a significant main effect of trial [F(9,396) = 5.064, p < 0.001], showing reducing symptom expectancies from the first trial to subsequent trials. This effect was further qualified by Trigger Information [F(9,396) = 3.066, p = 0.001], showing that this decline in symptom expectancies was specific for participants who had been informed of triggers indicating general vulnerability. The CS × Information interaction was not significant [F(1,22) = 0.88, p = 0.358], and although the CS × Trial × Information interaction did not reach significance [F(9,396) = 1.692, p = 0.089], visual inspection of this interaction suggested better differentiation for CS+/CS- symptom expectancies when participants had been given information about triggers as specific sensitivities vs. general vulnerability, (cf. Figure 1). Addition of CS category relationship to these analyses did not result in improved model fit (AIC increased from 4504 to 3552) or changes in observed significant effects.
Retention of Trigger Beliefs and Generalization to Novel Exemplars
For retention of trigger beliefs, we constructed a multilevel model that included fixed effects of CS (CS+ vs. CS-), CS novelty (old vs. new), Category Relationship (similar vs. different), and Trigger Information (general vulnerability vs. specific sensitivities), and included all interactions between these variables. The model also included a random (individual level) intercept, to account for the data being nested within participants. Results of this analysis is represented in Figure 2. In general, Symptom expectancy was greater for CS+ compared to CS- exemplars [F(1,858) = 29.094, p < 0.001]. Furthermore, symptom expectancy was greater for old compared to novel trigger exemplars [main effect of CS novelty: F(1,858) = 11.231, p = 0.001]. This effect was unmodulated by interactions with any of the other model factors, and we observed differential symptom expectancies both for old [t(858) = 3.812, p < 0.001] as well for novel [t(858) = 3.995, p < 0.001] category exemplars. Finally, we observed a significant CS × Category Relationship × Trigger Information 3-way interaction [F(1,858) = 4.174, p = 0.041]. Further exploration of this interaction showed significant differential CS+/CS- expectancies when information was given about general vulnerability and CS categories were more similar [t(858) = 4.075, p < 0.001] or when information about specific sensitivities was given and CS categories were more different [t(858) = 5.409, p < 0.001], differences between CS+/CS- for other combinations of Trigger Information and CS Category Relationship were non-significant (but in the expected direction, cf. Figure 2). We did not observe any other significant main effects or interactions in this analysis.
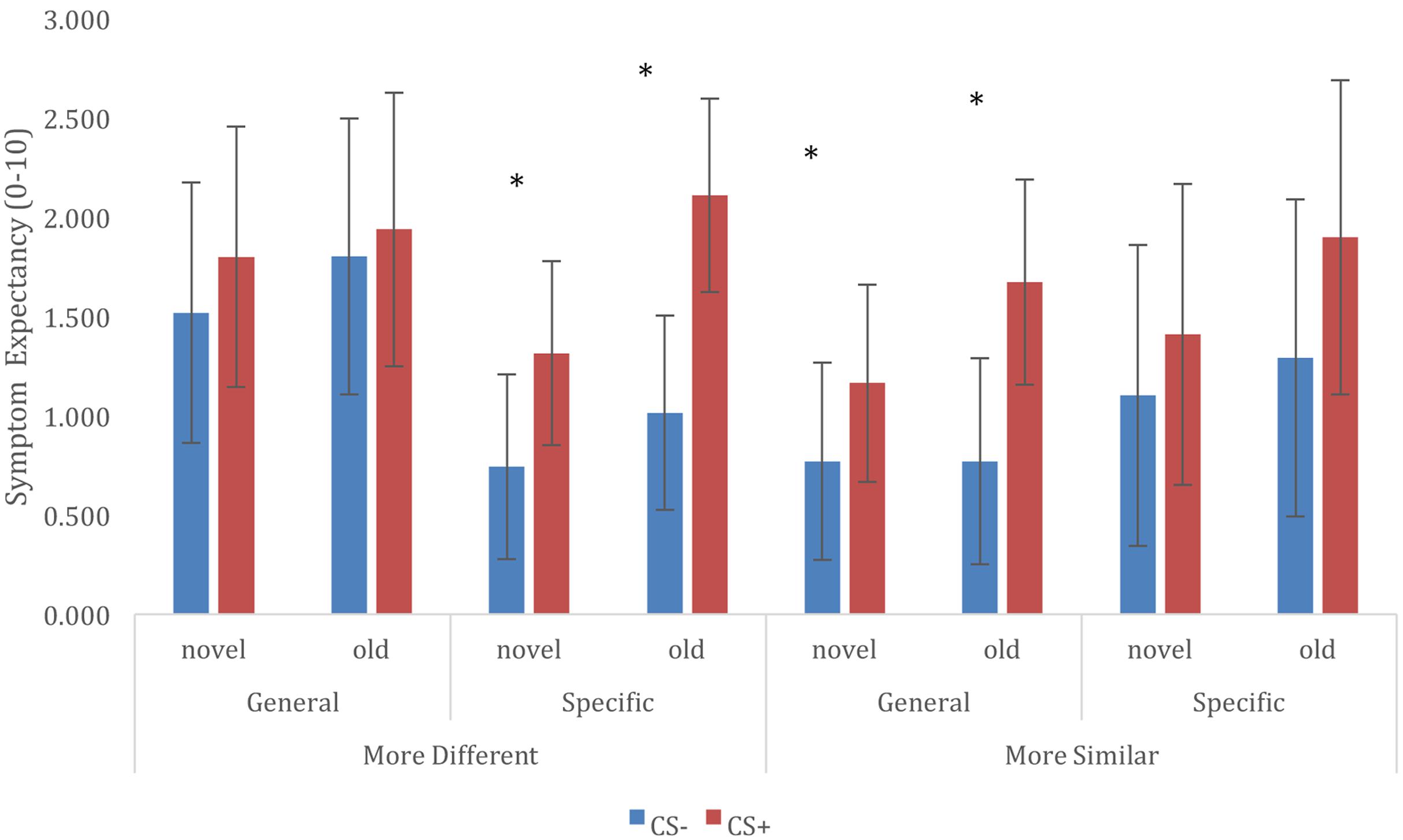
FIGURE 2. Retention of trigger-symptom expectancies, and generalization to novel trigger exemplars, depending on conditioned stimuli (CS), Trigger Information, and CS Category Relationship. ∗ CS+/CS– symptom expectancy differ at p < 0.05.
Generalization to Novel Trigger Categories
Based on the trigger categories that were used as CS+ and CS-, the novel trigger categories could be coded as G+ (related to CS+), G- (related to CS-) or Gu (unrelated to both CS categories). We constructed a multilevel model that included fixed effects of Stimulus Category (CS+, CS-, G+, G-, Gu), and Trigger Information (general vulnerability vs. specific sensitivities), and included all interactions between these variables. The model also included a random (individual level) intercept, to account for the data being nested within participants. Because of overlap of CS similarity with G categories (cf. Table 1), CS similarity was not added as a predictor to this model.
Results showed a main effect of Stimulus Category [F(4,1745) = 34.832, p < 0.001], further exploration of this effect showed that CS+ ratings were significantly higher compared to all other categories [CS+/CS-t(1738) = 7.824, p < 0.001; CS+/Gu t(1751) = 9.588, p < 0.001; CS+/G-t(1746) = 7.044, p < 0.001; CS+/G+ t(1746) = 7.011, p < 0.001], and that symptom expectancies for CS- exemplars were higher compared to Gu symptom expectancies [t(1751) = 3.329, p = 0.009], but not different from G+ and G- symptoms expectancies [CS-/G+ t(1746) = 1.165, p > 0.99; CS-/G-t(1746) = 1.198, p > 0.99]. Gu, G+, and G- ratings did not differ from each other (cf. Figure 3).
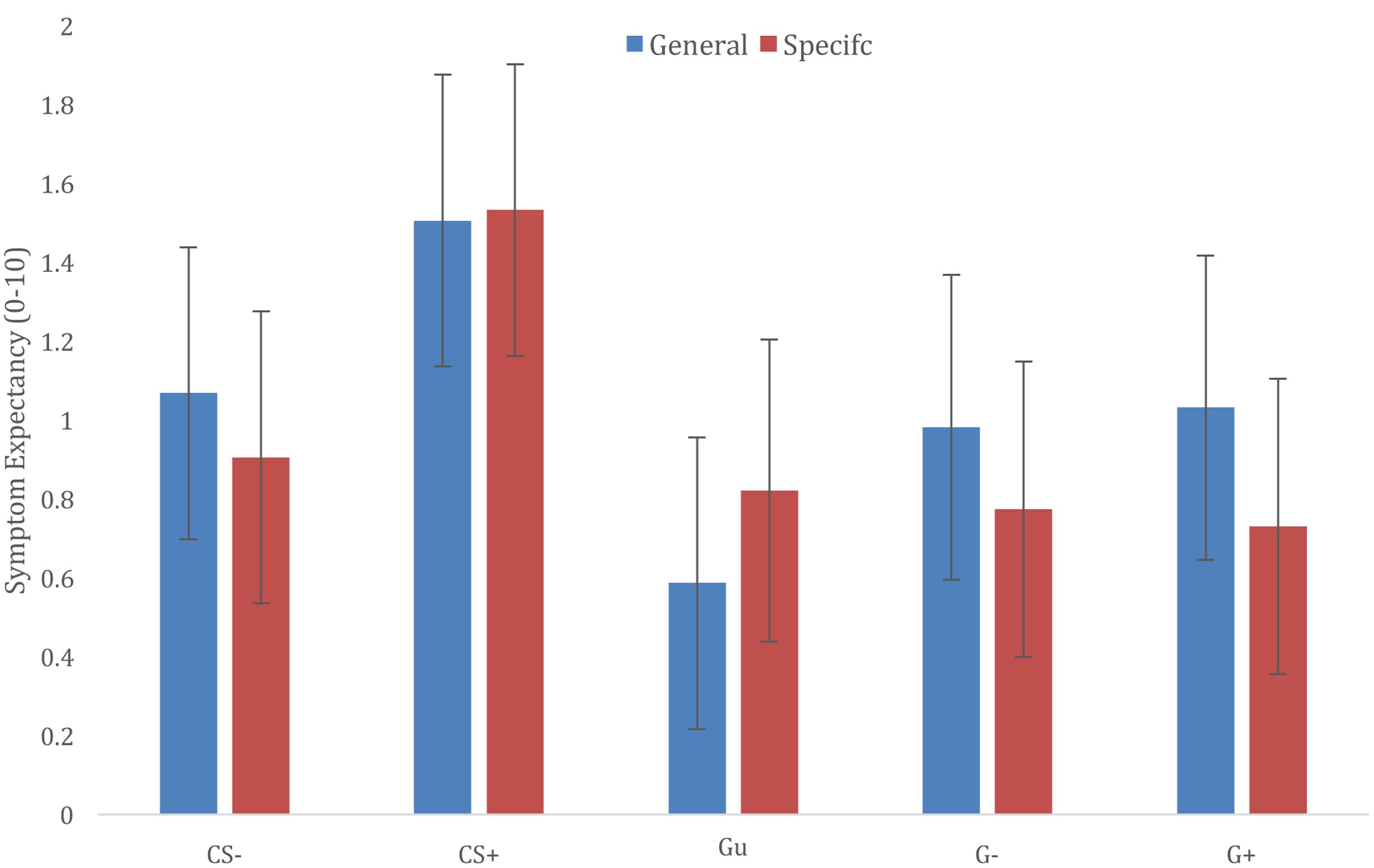
FIGURE 3. Generalization of trigger-symptom contingencies according to different information groups (general vulnerability vs. specific sensitivities).
The main effect of Trigger Information was not significant [F(1,22) = 0.025, p = 0.875], nor did the Trigger Information × Stimulus Category interaction yield a significant effect [F(4,1745) = 1.951, p = 0.100]. Visual exploration of the interaction suggested that providing information about specific sensitivities may prevent generalization to generalization categories that were related to CS categories (G+; G-), but not Gu category triggers (cf. Figure 3).
Discussion
In this experiment, we used a laboratory analog task in order to investigate the impact of information about the causal structure of asthma triggers and symptoms (asthma triggers being an indication of general vulnerability vs. specific sensitivities) on the acquisition, retention, and generalization of category-based trigger-symptom contingencies.
Results of the acquisition phase did not show clear evidence for the acquisition of category based symptom expectancies. This lack of clear acquisition effects is contrary to previous results with a similar experimental method, in a study that did not include explicit information about general vulnerability or specific sensitivities (Janssens et al., 2015). This may suggest that both types of information hinder the acquisition of differential trigger expectancies, although the large number of non-responders in the current experiment may limit the value of this comparison (cf. supra).
During the retention and generalization phase, we did observe retention of category based symptom expectancies, and generalization of these expectancies to novel CS+/CS- category exemplars. Information about specific sensitivities or general vulnerability had an impact on symptom expectancies, but this effect was not straightforward, as it was moderated by the trigger category relationship. Information about specific sensitivities led to better retention of differential expectancies when CS Categories had been more different, whereas information about general vulnerability led to better retention when CS Categories had been more similar. At first sight, the emergence of differential symptom expectancies after the acquisition phase may be puzzling. However, it is possible that the abstraction of category level information from the unique exemplars does not happen right away, and therefore would not show up on the trial by trial expectancy ratings. Furthermore, previous studies have shown category level consolidation effects, extending to other CS+ exemplars (Dunsmoor et al., 2015), which could explain why we do find differential CS+/CS- retention effects in absence clear differential learning during acquisition.
When confronted with novel (generalization) trigger categories, we could not confirm our hypothesis that trigger expectancies generalize to trigger categories that are related to the CS categories. However, participants did show some selectivity in generalization to novel categories, as evidenced by our finding that symptom expectancies for Gu triggers were lower than expectancies for CS+ and CS- triggers. Interestingly, we did not observe any differences between G+ and G- category exemplars, although the limited number of participants precludes us from making strong inferences about this. Generalization to novel categories was not moderated by our information manipulation, although visual inspection of the results was in line with information about asthma being caused by a general vulnerability leading to stronger symptom expectancies for trigger categories that were similar to CS+ or CS- categories, but not to potential triggers from unrelated categories.
Despite the many interaction effects that we observed, the results in the different conditions of our experiment demonstrate the impact of prior information on the acquisition, retention, and generalization of category-based trigger-symptom contingency beliefs. As the information conditions in our experiment mimic aspects of trigger-related information or advice that is given to patients by physicians or in internet-based asthma information, our findings may be of relevance to the management of asthma in daily life, as they suggest that experience-based beliefs about asthma triggers are shaped by prior information about asthma causality, as well as individual differences in symptom perception. The effects of prior information on generalization of trigger beliefs may be especially relevant, as they may help to explain the individual differences in asthma trigger beliefs that have been observed in individuals with asthma (Ritz et al., 2016) and associated differences in trigger avoidance strategies (Vernon et al., 2012).
Limitations
Our findings are limited by the observation that less than half of participants responded in a consistent way to our symptom induction of 60 s inhalation of an air mixture containing 7.5% CO2. Although previous studies had used longer inhalation periods (ranging from 90 s to 20 min) of 7.5% CO2 air mixtures in order to induce respiratory symptoms or symptoms of anxiety (Bailey et al., 2005; Bogaerts et al., 2005; Pappens et al., 2012; Janssens et al., 2015), our decision to use shorter duration symptom trials was motivated by a perceived need to reduce symptom burden (participation time), and did occur after pilot testing suggesting that participants were able to differentiate between 60 s room air and CO2 inhalation. Nevertheless, the results of this study show that longer periods of CO2 inhalation may be needed to reduce variability in symptom response and increase the differences between inhalation of a 7.5% CO2 air mixture and room air inhalation. Furthermore, even if participants reliably responded differently to the 7.5% CO2 air mixture and room air, they may not have picked up on these differences in a way that would lead them to form clear symptom-trigger contingencies. In our previous experiment using 90 s inhalation of the 7.5% CO2 air mixture, differentiation between CS+ and CS- symptom expectancies was markedly better.
Furthermore, our findings are limited in that we did not test behavioral outcomes related to these generalized trigger beliefs, nor did we test if these generalized triggers were sensitive to disconfirmation. However, studies on fear generalization have shown that generalization of negative outcome expectancies is accompanied by increased physiological manifestations of fear, as well as increased avoidance behavior to the generalization stimuli (van Meurs et al., 2014; Dymond et al., 2015), suggesting that generalized trigger-symptom contingencies can have an impact on trigger related behaviors. Nevertheless, future studies investigating effects of extinction on generalized trigger-symptom beliefs are needed to further gauge the impact that generalization can have in this domain.
A final limitation – as in many lab-based studies – is that design decisions that were aimed at improving internal validity may have reduced external validity of our experimental design. As noted in our previous study (Janssens et al., 2015), the use of uncommon allergens as experimental asthma triggers helps to isolate specific aspects of triggers as a potential basis of trigger acquisition and generalization, but these aspects may differ from the types of potential triggers that are experienced in real life. Similarly, the selection participants that do not have a history of allergy helps us to mimic conditions that parallel an early phase of asthma trigger identification, but may preclude generalization the lived and contextualized experience of individuals with asthma that may use a variety of information to infer trigger-symptom contingencies (Caress et al., 2002; Vernon et al., 2013).
conclusion
Our findings show that information about causality in asthma and knowledge about conceptual relationships between trigger categories influences the retention of category-based differential trigger-symptom expectancies, and generalization of these expectancies to novel trigger exemplars. Furthermore, retention and generalization of symptom expectancies was moderated by the similarity of CS+/CS- as well as similarities between CS and G categories. These findings underscore the role of higher order cognitions in contingency learning, and may help us to understand individual differences in asthma trigger beliefs that emerge over time. Finally, our findings suggest that pre-existing beliefs about asthma and asthma triggers may need to be taken into account when informing individuals with asthma about asthma trigger identification as an asthma management strategy, as these beliefs may impact subsequent learning of trigger-symptom contingencies in individuals with asthma.
Ethics Statement
This study was carried out in accordance with the recommendations of ethical guidelines of the American Psychological Association (APA) with written informed consent from all subjects. All subjects gave written informed consent in accordance with the Declaration of Helsinki. The protocol was approved by the Medical Ethical Committee of ‘University Hospitals Leuven.’
Author Contributions
TJ, IVD, and OVdB conceived the research questions and methodology, EC and TJ conducted the research and analyzed the data, EC, TJ, and OVdb contributed to writing the manuscript.
Funding
This research was funded by a Postdoctoral Fellowship awarded to TJ by the Flemish Research Foundation (Fonds Wetenschappelijk Onderzoek – Vlaanderen).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fpsyg.2017.00926/full#supplementary-material
References
Ahmed, O., and Lovibond, P. F. (2015a). The impact of instructions on generalization of conditioned fear in humans. Behav. Ther. 46, 597–603. doi: 10.1016/j.beth.2014.12.007
Ahmed, O., and Lovibond, P. F. (2015b). The impact of previously learned feature-relevance on generalisation of conditioned fear in humans. J. Behav. Ther. Exp. Psychiatry 46, 59–65. doi: 10.1016/j.jbtep.2014.08.001
Bailey, J. E., Argyropoulos, S. V., Kendrick, A. H., and Nutt, D. J. (2005). Behavioral and cardiovascular effects of 7.5% CO2 in human volunteers. Depress. Anxiety 21, 18–25. doi: 10.1002/da.20048
Bogaerts, K., Notebaert, K., Van Diest, I., Devriese, S., De Peuter, S., and Van den Bergh, O. (2005). Accuracy of respiratory symptom perception in different affective contexts. J. Psychosom. Res. 58, 537–543. doi: 10.1016/j.jpsychores.2004.12.005
Bousquet, J., Schünemann, H. J., Samolinski, B., Demoly, P., Baena-Cagnani, C. E., Bachert, C., et al. (2012). Allergic rhinitis and its impact on asthma (ARIA): achievements in 10 years and future needs. J. Allergy Clin. Immunol. 130, 1049–1062. doi: 10.1016/j.jaci.2012.07.053
Brough, H. A., Turner, P. J., Wright, T., Fox, A. T., Taylor, S. L., Warner, J. O., et al. (2015). Dietary management of peanut and tree nut allergy: what exactly should patients avoid? Clin. Exp. Allergy 45, 859–871. doi: 10.1111/cea.12466
Caress, A.-L., Luker, K., Woodcock, A., and Beaver, K. (2002). An exploratory study of priority information needs in adult asthma patients. Patient Educ. Couns. 47, 319–327. doi: 10.1016/S0738-3991(02)00005-8
Cnaan, A., Laird, N., and Slasor, P. (1997). Tutorial in biostatistics: using the general linear mixed model to analyse unbalanced repeated measures and longitudinal data. Stat. Med. 16, 2349–2380. doi: 10.1002/(SICI)1097-0258(19971030)16:20<2349::AID-SIM667>3.0.CO;2-E
Croft, D. R., and Peterson, M. W. (2002). AN evaluation of the quality and contents of asthma education on the world wide web∗. Chest 121, 1301–1307. doi: 10.1378/chest.121.4.1301
De Clerck, A., Verschuere, B., Crombez, G., and De Vlieger, P. (2006). Psychophysiological analysis (PSPHA): a modular script based program for analyzing psychophysiological data. Behav. Res. Methods 38, 504–510. doi: 10.3758/BF03192805
De Peuter, S., Lemaigre, V., Van Diest, I., and Van den Bergh, O. (2008). Illness-specific catastrophic thinking and overperception in asthma. Health Psychol. 27, 93–99. doi: 10.1037/0278-6133.27.1.93
Dunsmoor, J. E., Martin, A., and LaBar, K. S. (2012). Role of conceptual knowledge in learning and retention of conditioned fear. Biol. Psychol. 89, 300–305. doi: 10.1016/j.biopsycho.2011.11.002
Dunsmoor, J. E., Mitroff, S. R., and LaBar, K. S. (2009). Generalization of conditioned fear along a dimension of increasing fear intensity. Learn. Mem. 16, 460–469. doi: 10.1101/lm.1431609
Dunsmoor, J. E., and Murphy, G. L. (2015). Categories, concepts, and conditioning: how humans generalize fear. Trends Cogn. Sci. 19, 73–77. doi: 10.1016/j.tics.2014.12.003
Dunsmoor, J. E., Murty, V. P., Davachi, L., and Phelps, E. A. (2015). Emotional learning selectively and retroactively strengthens memories for related events. Nature 520, 345–348. doi: 10.1038/nature14106
Dymond, S., Dunsmoor, J. E., Vervliet, B., Roche, B., and Hermans, D. (2015). Fear generalization in humans: systematic review and implications for anxiety disorder research. Behav. Ther. 46, 561–582. doi: 10.1016/j.beth.2014.10.001
Engelen, U., De Peuter, S., Victoir, A., Van Diest, I., and Van den Bergh, O. (2006). Verdere validering van de “Positive and Negative Affect Schedule” (PANAS) en vergelijking van twee Nederlandstalige versies (Further validation of the positive and negative affect schedule (PANAS) and comparison of two Dutch versions). Gedrag Gezondheid 34, 89–102.
Global Initiative for Asthma (GINA) (2016). Global Strategy for Asthma Management and Prevention. Available at: http://ginasthma.org/wp-content/uploads/2016/04/wms-GINA-2016-main-report-final.pdf
Hagger, M. S., and Orbell, S. (2003). A meta-analytic review of the common-sense model of illness representations. Psychol. Health 18, 141–184. doi: 10.1080/088704403100081321
Halm, E. A., Mora, P., and Leventhal, H. (2006). No symptoms, no asthma: the acute episodic disease belief is associated with poor self-management among inner-city adults with persistent asthma. Chest 129, 573–580. doi: 10.1378/chest.129.3.573
Horne, R., and Weinman, J. (1999). Patients’ beliefs about prescribed medicines and their role in adherence to treatment in chronic physical illness. J. Psychosom. Res. 47, 555–567. doi: 10.1016/S0022-3999(99)00057-4
Huckvale, K., Car, M., Morrison, C., and Car, J. (2012). Apps for asthma self-management: a systematic assessment of content and tools. BMC Med. 10:144. doi: 10.1186/1741-7015-10-144
Janssens, T., and Harver, A. (2015). Effects of symptom perception interventions on trigger identification and quality of life in children with asthma. Pulm. Med. 2015:825137. doi: 10.1155/2015/825137
Janssens, T., Martens, F., Storms, N., and Van den Bergh, O. (2015). Generalization of respiratory symptom triggers. Behav. Ther. 46, 689–698. doi: 10.1016/j.beth.2015.05.003
Janssens, T., and Ritz, T. (2013). Perceived triggers of asthma: key to symptom perception and management. Clin. Exp. Allergy 43, 1000–1008. doi: 10.1111/cea.12138
Janssens, T., Verleden, G., De Peuter, S., Petersen, S., and Van den Bergh, O. (2011). The influence of fear of symptoms and perceived control on asthma symptom perception. J. Psychosom. Res. 71, 154–159. doi: 10.1016/j.jpsychores.2011.04.005
Janssens, T., Verleden, G., De Peuter, S., Van Diest, I., and Van den Bergh, O. (2009). Inaccurate perception of asthma symptoms: a cognitive-affective framework and implications for asthma treatment. Clin. Psychol. Rev. 8, 211–219. doi: 10.1016/j.cpr.2009.02.006
Kaptein, A. A., Klok, T., Moss-Morris, R., and Brand, P. L. (2010). Illness perceptions: impact on self-management and control in asthma. Curr. Opin. Allergy Clin. Immunol. 10, 194–199. doi: 10.1097/ACI.0b013e32833950c1
Leventhal, H., Meyer, D., and Nerenz, D. (1980). The common sense representation of illness danger. Contribut. Med. Psychol. 2, 7–30.
Li, J. T. C., Andrist, D., Bamlet, W. R., and Wolter, T. D. (2000). Accuracy of patient prediction of allergy skin test results. Ann. Allergy Asthma Immunol. 85, 382–384. doi: 10.1016/S1081-1206(10)62550-1
McQuaid, E. L., Howard, K., Kopel, S. J., Rosenblum, K., and Bibace, R. (2002). Developmental concepts of asthma: reasoning about illness and strategies for prevention. J. Appl. Dev. Psychol. 23, 179–194. doi: 10.1016/S0193-3973(02)00103-X
Pappens, M., De Peuter, S., Vansteenwegen, D., Van den Bergh, O., and Van Diest, I. (2012). Psychophysiological responses to CO2 inhalation. Int. J. Psychophysiol. 84, 45–50. doi: 10.1016/j.ijpsycho.2012.01.008
Peters, S. P., Jones, C. A., Haselkorn, T., Mink, D. R., Valacer, D. J., and Weiss, S. T. (2007). Real-world evaluation of asthma control and treatment (REACT): findings from a national web-based survey. J. Allergy Clin. Immunol. 119, 1454–1461. doi: 10.1016/j.jaci.2007.03.022
Rabe, K. F., Adachi, M., Lai, C. K. W., Soriano, J. B., Vermeire, P. A., Weiss, K. B., et al. (2004). Worldwide severity and control of asthma in children and adults: the global asthma insights and reality surveys. J. Allergy Clin. Immunol. 114, 40–47. doi: 10.1016/j.jaci.2004.04.042
Ritz, T., Steptoe, A., Bobb, C., Harris, A. H. S., and Edwards, M. (2006). The asthma trigger inventory: validation of a questionnaire for perceived triggers of asthma. Psychosom. Med. 68, 956–965. doi: 10.1097/01.psy.0000248898.59557.74
Ritz, T., Wittchen, H.-U., Klotsche, J., Mühlig, S., and Riedel, O. (2016). Asthma trigger reports are associated with low quality of life, exacerbations, and emergency treatments. Ann. Am. Thorac. Soc. 13, 204–211. doi: 10.1513/AnnalsATS.201506-390OC
Smith, H., Gooding, S., Brown, R., and Frew, A. (1998). Evaluation of readability and accuracy of information leaflets in general practice for patients with asthma. BMJ 317, 264–265. doi: 10.1136/bmj.317.7153.264
Smith, H. E., Hogger, C., Lallemant, C., Crook, D., and Frew, A. J. (2009). Is structured allergy history sufficient when assessing patients with asthma and rhinitis in general practice? J. Allergy Clin. Immunol. 123, 646–650. doi: 10.1016/j.jaci.2008.11.005
Spruyt, A., Clarysse, J., Vansteenwegen, D., Baeyens, F., and Hermans, D. (2010). Affect 4.0: a free software package for implementing psychological and psychophysiological experiments. Exp. Psychol. 57, 36–45. doi: 10.1027/1618-3169/a000005
Trollvik, A., and Severinsson, E. (2004). Parents’ experiences of asthma: process from chaos to coping. Nursing Health Sci. 6, 93–99. doi: 10.1111/j.1442-2018.2004.00179.x
Van Diest, I., Smits, D., Decremer, D., Maes, L., and Claes, L. (2010). The Dutch claustrophobia questionnaire: psychometric properties and predictive validity. J. Anxiety Disord. 24, 715–722. doi: 10.1016/j.janxdis.2010.05.003
van Meurs, B., Wiggert, N., Wicker, I., and Lissek, S. (2014). Maladaptive behavioral consequences of conditioned fear-generalization: a pronounced, yet sparsely studied, feature of anxiety pathology. Behav. Res. Ther. 57, 29–37. doi: 10.1016/j.brat.2014.03.009
Vernon, M. K., Bell, J. A., Wiklund, I., Dale, P., and Chapman, K. (2013). Asthma control and asthma triggers: the patient perspective. J. Asthma Allergy Educ. 4, 155–164. doi: 10.1177/2150129713483307
Vernon, M. K., Wiklund, I., Bell, J. A., Dale, P., and Chapman, K. R. (2012). What do we know about asthma triggers? A review of the literature. J. Asthma 49, 991–998. doi: 10.3109/02770903.2012.738268
Vervliet, B., and Geens, M. (2014). Fear generalization in humans: impact of feature learning on conditioning and extinction. Neurobiol. Learn. Mem. 113, 143–148. doi: 10.1016/j.nlm.2013.10.002
Vervliet, B., Kindt, M., Vansteenwegen, D., and Hermans, D. (2010). Fear generalization in humans: impact of verbal instructions. Behav. Res. Ther. 48, 38–43. doi: 10.1016/j.brat.2009.09.005
von Leupoldt, A., and Dahme, B. (2012). Looking at allergens increases symptom report in patients with allergic asthma. J. Asthma 49, 1027–1029. doi: 10.3109/02770903.2012.733994
Keywords: asthma triggers, contingency learning, generalization (psychology), expectancy violation, illness perceptions
Citation: Janssens T, Caris E, Van Diest I and Van den Bergh O (2017) Learning to Detect Triggers of Airway Symptoms: The Role of Illness Beliefs, Conceptual Categories and Actual Experience with Allergic Symptoms. Front. Psychol. 8:926. doi: 10.3389/fpsyg.2017.00926
Received: 27 October 2016; Accepted: 19 May 2017;
Published: 07 June 2017.
Edited by:
Anna Thorwart, Philipps University of Marburg, GermanyReviewed by:
Gabrielle Weidemann, Western Sydney University, AustraliaMitchell Rabinowitz, Fordham University, United States
Copyright © 2017 Janssens, Caris, Van Diest and Van den Bergh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Thomas Janssens, thomas.janssens@kuleuven.be
 Thomas Janssens
Thomas Janssens Eva Caris
Eva Caris Ilse Van Diest
Ilse Van Diest Omer Van den Bergh
Omer Van den Bergh