Comparison of the Gait Biomechanical Constraints in Three Different Type of Neuromotor Damages
- 1Department of Neurorehabilitation and Robotics, Movement Analysis and Robotics Laboratory (MARLab), “Bambino Gesù” Children’s Hospital, IRCCS, Rome, Italy
- 2Department of Biomedicine and Prevention, University of Rome “Tor Vergata”, Rome, Italy
- 3Department of Systems Medicine, University of Rome “Tor Vergata”, Rome, Italy
Background and Objective: Absolute angle represents the inclination of a body segment relative to a fixed reference in space. This work compares the absolute and relative angles for exploring biomechanical gait constraints.
Methods: Gait patterns of different neuromotor conditions were analyzed using 3D gait analysis: normal gait (healthy, H), Cerebral Palsy (CP), Charcot Marie Tooth (CMT) and Duchenne Muscular Dystrophy (DMD), representing central and peripheral nervous system and muscular disorders, respectively. Forty-two children underwent gait analysis: 10 children affected by CP, 10 children by CMT, 10 children by DMD and 12 healthy children. The kinematic and kinetic parameters were collected to describe the biomechanical pattern of participants’ lower limbs. The absolute angles of thigh, leg and foot were calculated using the trigonometric relationship of the tangent. For each absolute series, the mean, range, maximum, minimum and initial contact were calculated. Kinematic and kinetic gait data were studied, and the results were compared with the literature.
Results: Statistical analysis of the absolute angles showed how, at the local level, the single segments (thigh, leg and foot) behave differently depending on the pathology. However, if the lower limb is studied globally (sum of the kinematics of the three segments: thigh, leg and foot), a biomechanical constraint emerges.
Conclusion: Each segment compensates separately for the disease deficit so as to maintain a global biomechanical invariance. Using a model of inter-joint co-variation could improve the interpretation of the clinical gait pattern.
Introduction
The introduction of gait analysis has enhanced the concept that motor behavior emerges from the body-environment coupling (Kuniyoshi and Suzuki, 2004; Abu-Faraj et al., 2015). Gait analysis is a multi-factorial analysis assessing kinematic, kinetic and electromyographic activities (EMG). These techniques are used to identify the kinematic determinants of human locomotion. Attention was focused on the relationship between the biomechanics of the human body and the brain (Lacquaniti et al., 1999) as these two elements do not work at the same logical level. The body biomechanics represents a given system of constraints, as shown by Collins et al. (2005) with his bipedal modeling of dynamic passive gait. In living creatures, the body is characterized by a redundancy of degree of freedom (DoF), raising the problem about their control during dynamic functional activities (Davies, 1968). The Central Nervous System fine-tunes the synergic actions of the muscles controlling the redundancy of the body DoF (Davies, 1968). In this perspective, the brain is the “medium” of the relationship between the organism and the environment, as explained by the ecologic approach to motor control and learning (Gibson, 1979). Although this relationship is well established, knowledge concerning the particular strategies used by the brain to mediate between body biomechanics and the environment is limited. The nature of the biomechanical constraints implies that appropriate solutions for walking are limited, and the usual bipedal walking represents a sort of final common path. However, different gait patterns that deviate from the “typical” one are frequently observed in pathologic conditions. It is possible to hypothesize that the way of walking, expressed in pathological conditions, can offer some keys for understanding the invariance rules that bind the biomechanical and neurological mechanisms. As an example, it was demonstrated that patients with hemiplegia and voluntary toe walkers shared the same kinematic, kinetic and EMG patterns (Romkes and Brunner, 2007). To explore the elements of invariance, we decided to analyze the gait pattern of children with different pathologies looking at differences and similarities. We analyzed the gait pattern that emerged from three different types of damage to the neuromotor system caused by Cerebral Palsy (CP), Charcot-Marie-Tooth (CMT) and Duchenne Muscular Dystrophy (DMD). They can be considered paradigmatic examples corresponding to damage involving the upper motor neuron, the lower motor neuron, and the muscles. CP, CMT and DMD children present complex and heterogeneous gait patterns already described using gait analysis tools (Salami et al., 2017; Wojciechowski et al., 2017; Davids et al., 2018; Goudriaan et al., 2018; Romano et al., 2019). To date, there has been no comparative study aimed at defining similarities and differences. The present study was facilitated by the fact that our gait lab is situated within a big clinical research center specialized in following children with rare diseases.
Briefly, CP is characterized by movement disorder, spasticity, muscle weakness, ataxia and rigidity (Armand et al., 2016). It has been reported that the alterations in the selective motor control are the result of failure to control reciprocal activation of the agonist and the antagonist muscles and correlate with the gait inability (Crenna, 1998; Chruscikowski et al., 2017). Their gait alterations are currently classified in literature (Rodda et al., 2004; Cioni et al., 2008) without reaching a consensus.
CMT is a peripheral nervous system disorder. Affected patients show skeletal deformities, distal muscle weakness and atrophy, and sensory impairment leading to walking impairment (Lencioni et al., 2017) which have already been classified in literature (Wojciechowski et al., 2017).
DMD is characterized by a progressive replacement of muscle fibers with fibro-fatty tissue and severe muscular weakness. Progressive muscular degeneration determines the onset of compensatory strategies during walking (Doglio et al., 2011). The gait pattern of this population has been widely described (Sienko Thomas et al., 2010; Goudriaan et al., 2018).
The present study aimed to investigate similarities and differences between the gait patterns of three different pathologic groups of children with CMT, DMD and CP compared to healthy children. The main hypothesis is that the three different gait patterns were influenced by the particular nature of the pathology (central, peripheral and muscular). However, they could also share elements of invariance. This invariance can be induced by body biomechanics and environmental physics, similarly to what identified in studies of lower limb absolute angles in healthy subjects (Borghese et al., 1996; Hamill et al., 2014). Ours is a pilot study in which, besides kinematics, the kinematic gait relative and absolute body segment configurations were compared, searching for differences and similarities, to enhance present knowledge related to the control of bipedal locomotion in pathological conditions.
Materials and Methods
Participants
Ten children with CMT, ten children with DMD and ten children with CP were enrolled in the study. The eligibility criteria for this study were: age between 5 and 15 years and independent walking without orthosis. CP is a heterogeneous condition by definition. Consequently, both to reduce CP heterogeneity and to achieve the aims of this study, only participants with Gross Motor Function Classification System I and II were included, with a gait pattern corresponding to group IV of the Rodda et al. (2004) classification (Rodda et al., 2004) and to form IV of the Cioni et al. (2008) gait classification (Cioni et al., 2008). Exclusion criteria were: surgical treatment in the last year and administration of botulin toxin or experimental drug during the previous 6 months.
The group of children with CMT consisted of eight boys and two girls, with an average age of 12.0 (range: 7.0–15.0 years), an average weight of 46.3 (range: 25.0–79.5 kg), an average height of 1.44 (range:1.19–1.69 m) and an average leg length of 0.76 (range: 0.64–0.96 m).
The group of children with DMD consisted of 10 boys, with an average age of 7.7 (range: 5.0–11.0 years), average weight of 27.9 (range: 17.8–46.0 kg), average height of 1.23 (range: 1.04–1.63 m) and average leg length of 0.61 (range: 0.53–0.71 m).
The group of children with CP consisted of five boys and five girls, with an average age of 9.3 (range: 5.6–15.7 years), an average weight of 29.0 (range: 18.5–49.5 kg), an average height of 1.28 (range: 0.96–1.57 m) and average leg length of 0.69 (range: 0.48–0.87 m).
Reference data were collected in a group of twelve children, seven boys and five girls, without any neurological or neuromuscular problems, with an average age of 10.9 (range: 6.0–14.5), an average weight of 39.2 (range: 22.0–60.3 kg), average height 1.43 (range: 1.15–1.68 m) and average leg length 0.79 (range: 0.63–0.94 m).
All the children and their parents gave informed consent before starting the evaluation sessions. The Ethics Committee of the Hospital authorized the study. For an exhaustive clinical description of the pathologic groups see Table 1.
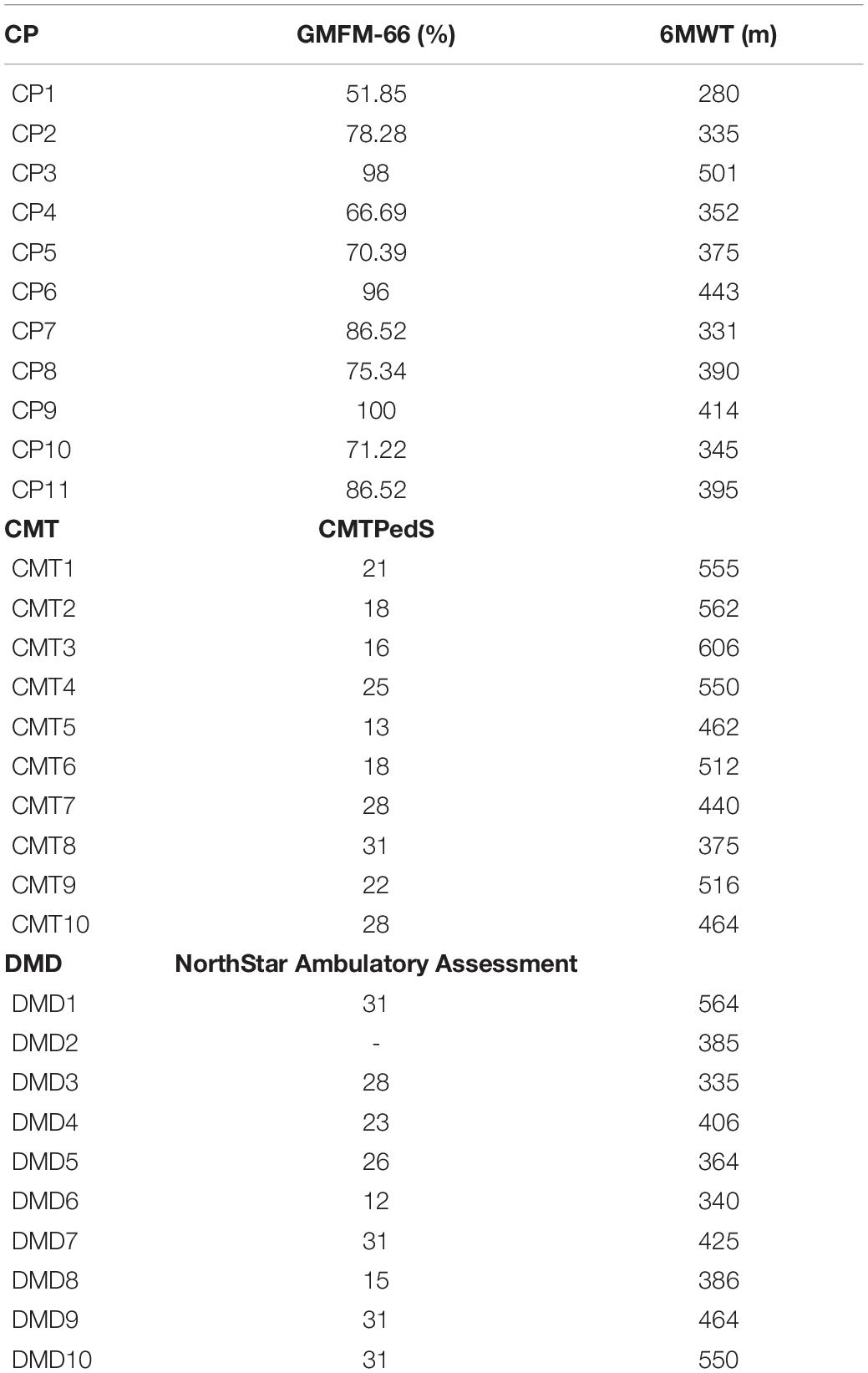
Table 1. Clinical scale and six Minute Walking Test (6MWT): For Cerebral Palsy (CP) the clinical scale used is the Gross Motor Function Measure-66 (GMFM-66), for Charcot Marie Tooth (CMT) the clinical scale is the CMT Pediatric Scale (CMTPedS), and for Duchenne Muscular Dystrophy (DMD) the clinical scale is the NorthStar Ambulatory Assessment (NSAA).
Gait Analysis
Gait analysis was performed using an eight-camera motion capture system (Vicon MX, United Kingdom) with sampling rates of 200 Hz and two force plates (AMTI, Or6-6, United States) with sampling rates of 1 kHz. The two force plates were situated in the middle portion of a 10 m walkway. Plug-in-Gait protocol for reconstructing a body kinematic and kinetic model was used. Participants walked barefoot at their self-selected speed. For each child, three representative gait cycles were considered. Kinematic and kinetic temporal series were normalized to the stride duration. Kinetic data were normalized to the subject’s weight. In addition, we evaluated spatio-temporal parameters, walking velocity and step length were normalized to leg length.
The lower limb absolute angles (segment angles) that describe the segment’s orientation in space were also calculated. Absolute angles are computed using the trigonometric relationship of the tangent. The tangent is equal to the angular coefficient obtained with a linear fit between the proximal endpoints of the segment. Schematic illustrations of the absolute angles (thigh, leg and foot) and relative angles (hip, knee and ankle) are reported in Figure 1. The following list of variables was selected from absolute angle curves of thigh, leg and foot: initial contact, average, range, maximum and minimum. The sum of the absolute angles (thigh, leg and foot) was then evaluated to determine the biomechanical constraints in lower limbs. The initial contact, average, range, maximum and minimum were calculated from the total absolute angle. See Tables 2, 3 for a detailed report of the kinematic and kinetic parameters.
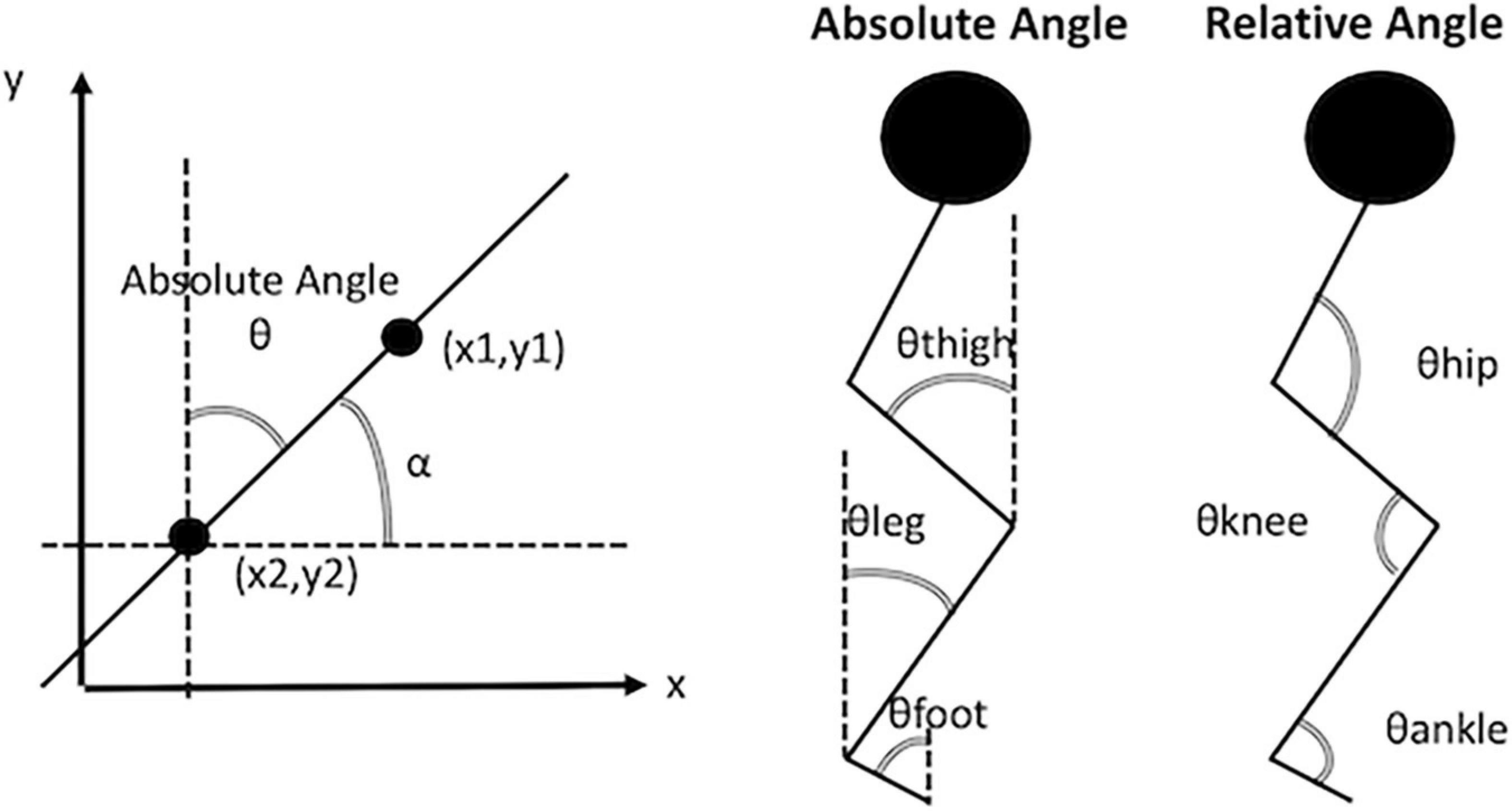
Figure 1. Illustrative example of absolute angles and relative angles. In the panel (right) the absolute angle ⊖ is the complementary angle of angle α. The angle α is obtained from the arctangent of the slope of the line passing through the points (x1, y1) and (x2, y2). In the panel (center) are depicted the absolute angles of thigh, leg, and foot. In the panel (left) are depicted the relative angle of the hip, the knee and the ankle.
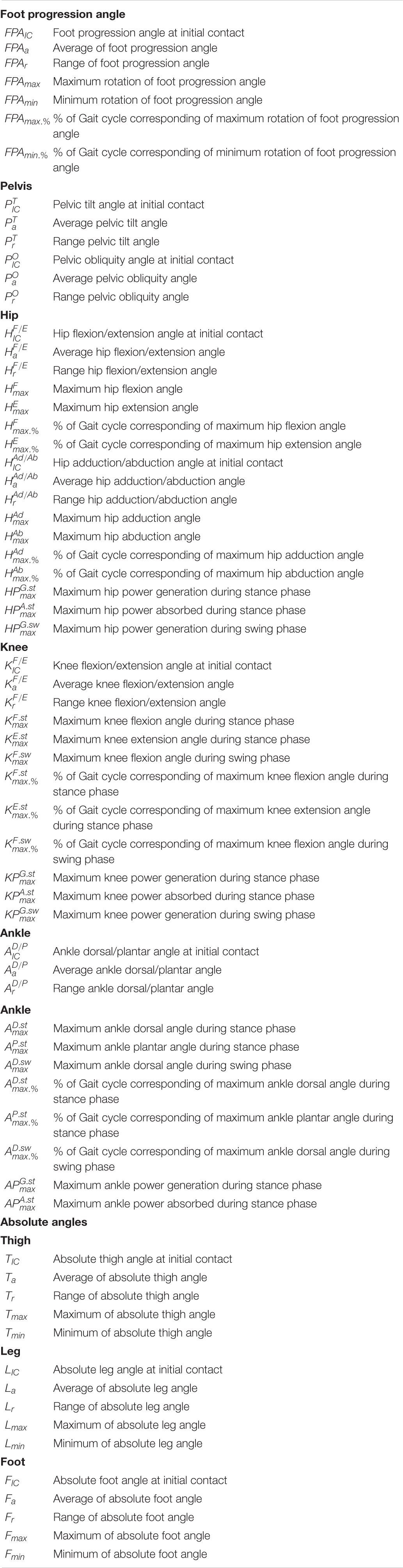
Table 2. The list of the kinematic and kinetic variables of the lower limb (pelvis, hip, knee and ankle) and the absolute angles of thigh, leg and foot.

Table 3. Differences between the median values of two different pathologies: Healthy (H), Cerebral Palsy (CP), Charcot Marie Tooth (CMT) and Duchenne Muscular Dystrophy (DMD).
Statistics
For each child, we evaluated three kinematic and three kinetic parameters for each representative gait cycle. Finally, we calculated the mean values. The Shapiro–Wilk normality test was used to verify the normal distribution. Since the data were not normally distributed, we assessed a non-parametric statistic. The Wilcoxon signed rank test for paired samples was used to compare the data obtained for the left and right limbs. Since the test was not statistically significant, the average between right and left limbs was calculated for all patients. The Kruskal–Wallis test and a post–hoc with Bonferroni correction determined significant differences in the kinematic (relative and absolute angles) and kinetic parameters between the four groups (CMT, DMD, CP and healthy). A p < 0.05 was considered to indicate statistical significance.
Results
Spatio-temporal, kinematic (relative and absolute angles) and kinetic parameters for the variable illustrated in Table 2 are shown in Table 3.
Children with DMD walked with a statistically significant higher stride velocity and a shorter stride time than those with CMT and CP. Stride width values were significantly larger in DMD and CP children compared to healthy controls. No statistically significant differences in any spatio-temporal parameters were found when comparing the children with CMT with the healthy children or those with CP (Table 3).
The analysis of kinematic pelvic parameters did not show statistically significant differences between healthy controls and children with CMT or DMD (Figure 2). The children with CP presented average and range pelvic tilt angles statistically higher than controls and children with CMT. Although we did not find any statistical significance in this sample, we observed that patients with DMD tended toward a pelvic anteversion and CMT patients toward a pelvic retroversion, as it is shown in the sagittal view of the Pelvis angle in Figure 2 (i.e. the CMT time series angle is the lower curve).
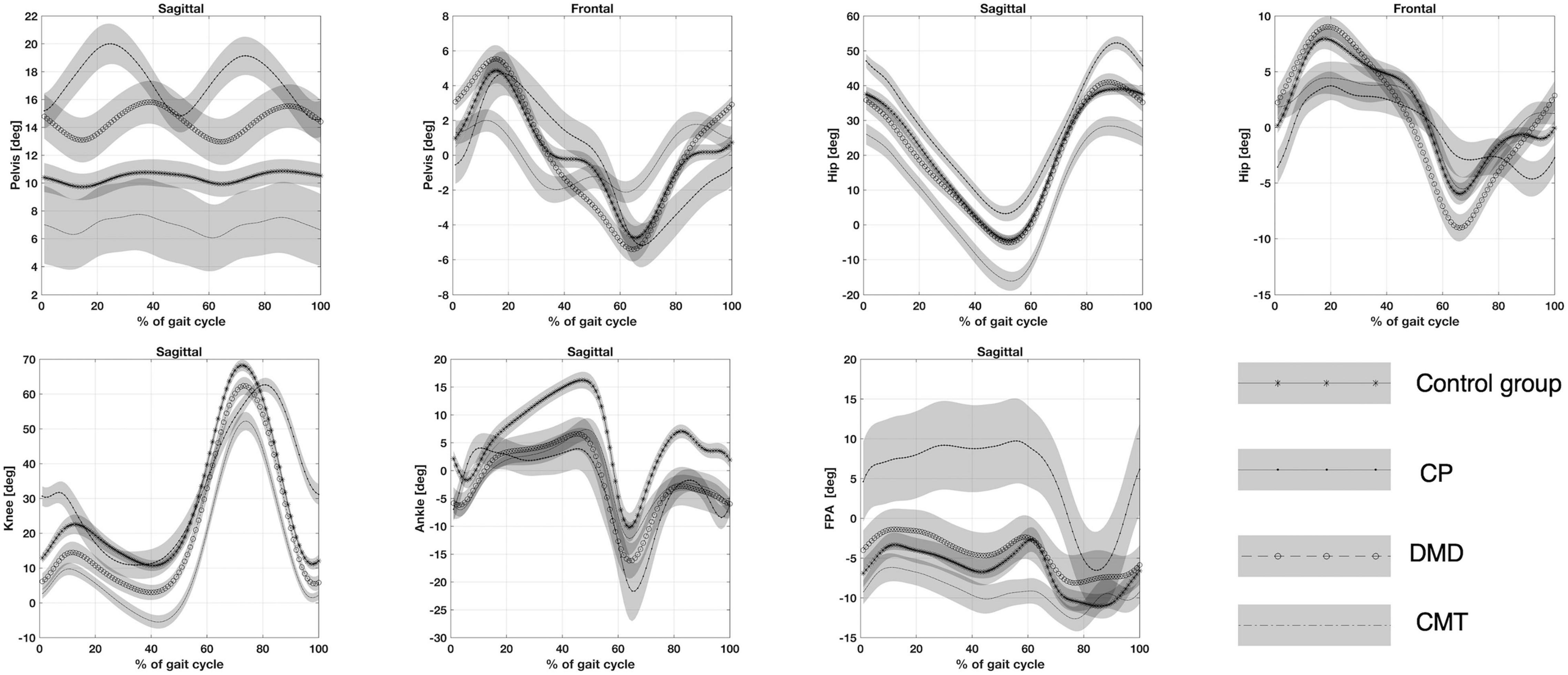
Figure 2. Top panel (from left to right): Pelvis angle on the sagittal and the frontal plane. Hip angle on the sagittal and the frontal plane. Bottom panel (from left to right): Knee angle, Ankle angle on the sagittal plane and Foot progression angle (FPA) on the transversal plane. CP, participants with Cerebral Palsy; CMT, participants with Charcot Marie Tooth; DMD, participants with Duchenne Muscular Dystrophy; normal gait (healthy, H).
The pelvic obliquity angle at initial contact in children affected by DMD was increased compared to those with CP. The children with CMT showed a reduction in the range of the pelvic obliquity angle compared to DMD and CP groups (Figure 2).
The children with CMT presented the average of the flexion/extension and maximum extension of hip angle lower compared to healthy children (Figure 2). The CP group showed an increase in maximum hip flexion compared to healthy patients. The children affected by CMT showed initial contact, average, and maximum and minimum flexion/extension of the hip angles statistically lower compared to CP children. For the patients affected by CP the initial contact of hip adduction/abduction angle resulted lower and statistically significant when compared to children with DMD and CMT (Figure 2). Patients with DMD showed a range of hip adduction/abduction angle greater than peers with CMT and CP.
The children with CMT showed decreased initial contact, average and maximum and minimum knee flexion angles compared to healthy children (Figure 2). However, the initial contact, average, and maximum knee flexion angles were significantly higher in children with CP than in peers with CMT and DMD.
Ankle dorsal/plantar angle at initial contact, average and maximum ankle dorsal angle during the stance phase were significantly lower in participants with CMT and DMD than in healthy children (Figure 2). The maximum ankle dorsal angle during swing phase values was statistically lower for all pathological groups (CMT, DMD and CP) than for healthy control groups. The ankle dorsal/plantar angle range resulted lower for children with CMT compared to participants with CP.
Increased range and maximum foot progression angles were only observed in children with CP compared with healthy children (Figure 2).
The children with CP presented initial contact, average, range, and maximum and minimum rotation of the foot progression angles significantly higher than children with CMT (Figure 2). We observed a reduction of the range of the foot progression angle for children with DMD compared to children with CP.
The absolute angle for thigh, leg and foot are shown in Figure 3. There are no statistically significant differences between CMT and DMD groups for absolute angle of thigh, leg and foot. However, there were differences between the children with CMT and peers in the CP and healthy groups for the thigh and leg segments but not for the foot segment. Differences between children in the DMD and CP groups were found for the thigh and leg segments. Patients in the DMD group only differ from the healthy group as regards the thigh segment (Table 2). However, Figure 3 shows that the sum of the absolute angles (thigh, leg and foot) does not present statistically significant differences between these groups (p > 0.05).
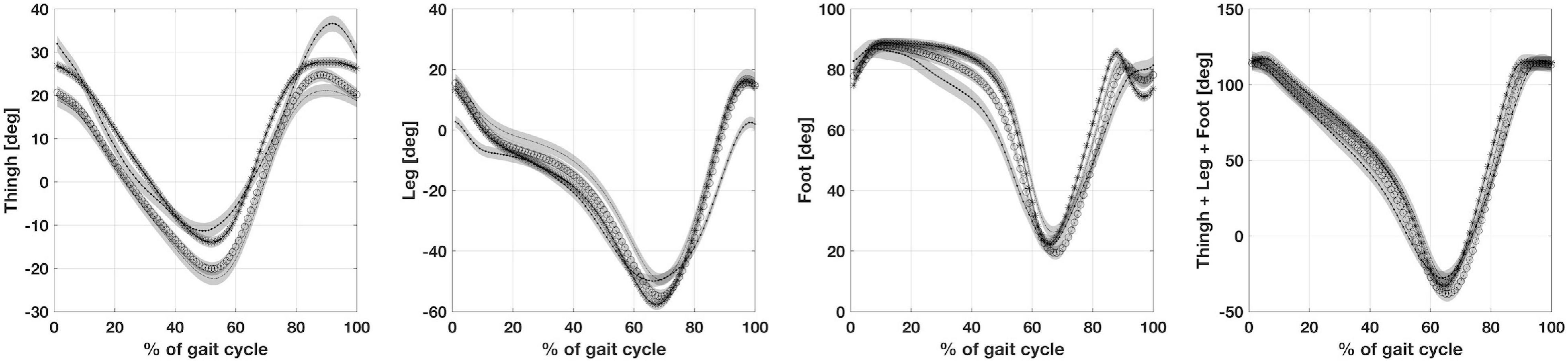
Figure 3. Illustrates from left to right, the absolute angles of thigh, leg, foot and the sum of thigh, leg and foot. CP, participants with Cerebral Palsy; CMT, participants with Charcot Marie Tooth; DMD, participants with Duchenne Muscular Dystrophy; normal gait (healthy, H).
Patients with CP showed an increase in maximum hip power generation during the stance phase when compared to peers with CMT and DMD. Maximum knee power absorbed during stance was lower in children with CP than in participants with CMT and DMD. The children with CP showed maximum ankle power generation and absorption during the stance phase statistically lower than healthy children (Figure 4).
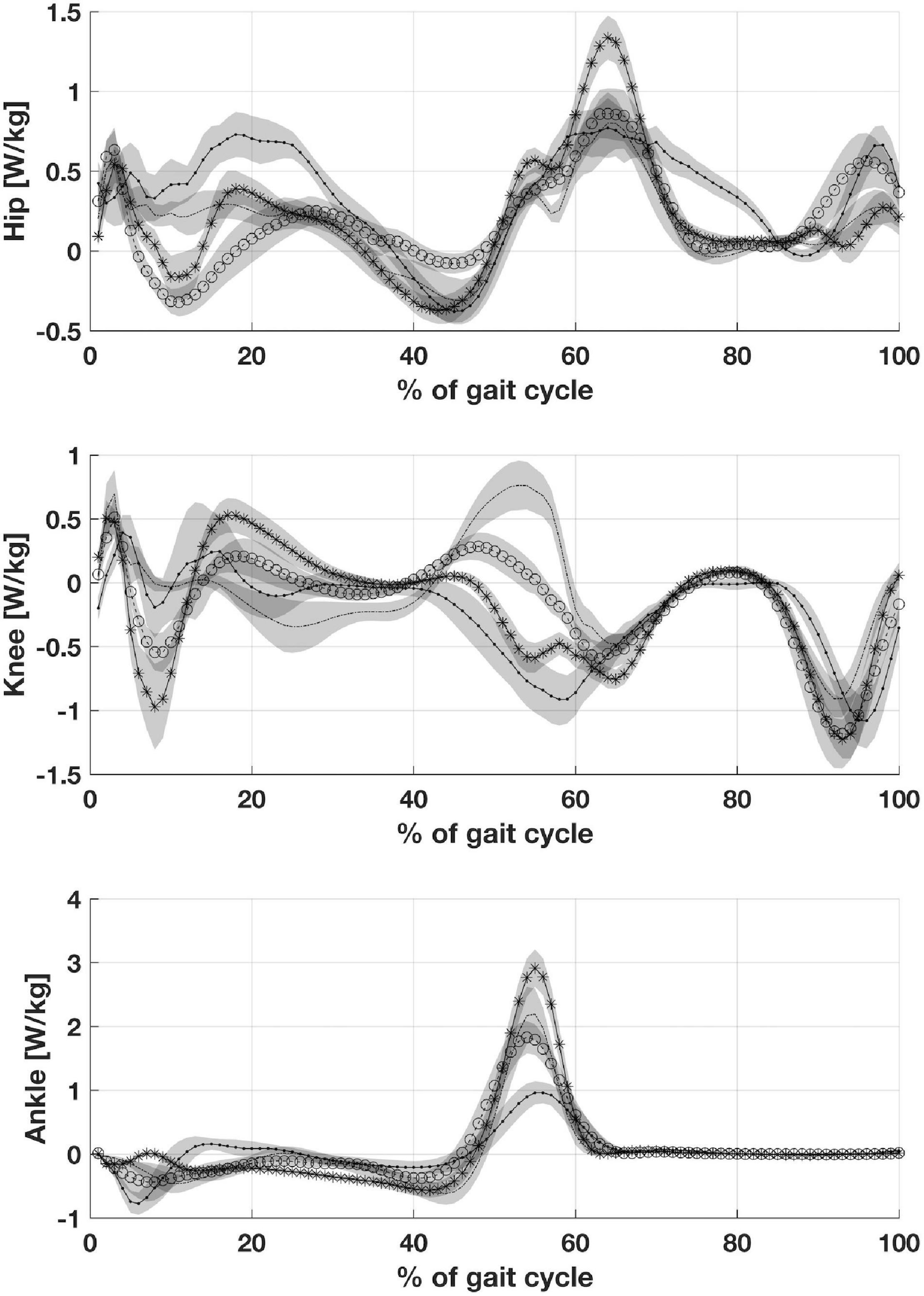
Figure 4. Depicts joints power: Hip joint power, Knee joint power and Ankle joint power. CP, participants with Cerebral Palsy; CMT, participants with Charcot Marie Tooth; DMD, participants with Duchenne Muscular Dystrophy; normal gait (healthy, H).
Discussion
In the present study, we conducted gait analysis on children affected by three different pathologies (CP, CMT, and DMD), representing paradigmatic examples corresponding to damage mainly involving the upper motoneuron, the lower motoneuron and the muscles, respectively. We analyzed spatial and temporal parameters, kinematics and kinetics of gait in children with CMT, DMD, and CP compared to a control group. The stride velocity of the three pathologies (CMT, DMD and CP) was not statistically significant compared to the healthy group, which allows us to underline how the three groups are comparable from the functional perspective.
We studied kinematics, analyzing lower limb relative and absolute angles with respect to the vertical axis. The study of relative angles evidenced the distinctive solutions peculiar to each pathologic group; at the same time, the study of absolute angles highlighted the crucial role of the pelvis and foot segments with respect to the typical pattern shown by the thigh and the shank.
Furthermore, it is noteworthy that standard gait analysis reports merge absolute segment orientation in space, like the pelvis or the foot progression angle, with relative joint angles of the hip, knee, and ankle. Another interesting aspect is the special functional role of some body regions: the pelvis embodies the relationship between the lower limbs and the upper body (Amori et al., 2015); and the foot relates the body to the environment.
We observed increasing hip flexion with the increase of pelvis anteversion and vice versa. The absolute pelvis orientation in space and the hip angles are connected. At the same time, the knee mediates the attitude of the pelvis and the foot, reducing the range of motion as in the case of reduction of ankle range in conjunction with the pelvis anteversion attitude (Figure 2). Furthermore, the knee angle tended to shift in flexion or extension in relation to pelvis anteversion or retroversion, respectively. The above-mentioned pathological factor restricts the biomechanical relationship to a particular path. It is impossible to distinguish the contributions of two elements: biomechanics on one side and pathological conditions on the other, in both their central and peripheral expressions (Crenna, 1998). Children with CP that are affected mainly by selective motor control deficits and muscle weakness showed a marked reduction of dorsal flexion in stance and increased plantar flexion in pre-swing. This behavior allows storage and release of the mechanical energy in the muscle structure, conserving energy on the vertical plane (Holt et al., 2000; Fonseca et al., 2001). Children with Duchenne dystrophy showed similar ankle behavior. Yet, in that case, even if the mechanism of passive mechanical energy exploitation is similar, the cause is different because it is due to the decline of contractile fiber in muscles and their relative weakness (Romano et al., 2019). In children with Charcot-Marie-Tooth, we observed a reduction of the ankle range of motion, probably linked mainly to both muscular and articular degeneration. In all the cases, the specific anatomical changes represent constraining elements for gait function.
It is possible to explain the differences in pelvis behavior if we consider the previously mentioned factors in mediation with the upper body. The balancing of the pelvis implies a fine-tuning of muscle activities to stabilize the segment on the two spherical hip joints in a dynamic equilibrium compatible with gait progression. Pelvic anteversion in children with CP could be interpreted as a simplification of the pelvis stabilization in stance phase, hanging on the hamstring muscles, reaching maximum anteversion during the monopodic support gait phase. In children with Duchenne, where the main problem is the weakness and not the deficit of fine motor control, the double bump is present, but it is in phase with the control group, a sort of boost of functional activities (Romano et al., 2019). Children with Charcot-Marie-Tooth present a tendency toward pelvic retroversion. It is possible to observe similar attitudes in the gait of children who are blind from birth (Gazzellini et al., 2016). It is an attitude linked with a cautious gait in which the dynamic aspects are restrained. The causes are dissimilar, that is, in blind children, the uncertainty stems from the reduction of exteroception information, while in the children studied here, it is due to the decrease of information from the foot engaging the terrain. What is common to the two situations is the absence of information from a specific sensory channel. We can speculate about the role of another element which may influence these gait differences: perception. The absolute angles of thigh, leg and foot showed a more consistent behavior throughout the different pathologies. Only children with CP differentiated from the other groups during the final swing phase for the hip and during stance for the foot.
It is intriguing to note that if we sum the thigh and the leg orientation with respect to the vertical axis in the three disease groups, any differences between the three pathologies are canceled. We can hypothesize that these time series represent the invariance necessary to achieve an efficient gait, using all the peculiar available resources, both central and peripheral. When all the lower limb segments were considered together, a slight variation during stance induced by the foot orientation differentiates mainly children with CP from children with CMT. The characteristics of the foot condition in these two pathologies resulted from central and peripheral disease, respectively. The nature of these pathologies leads to opposite feet musculoskeletal abnormalities, which influence the strike of the foot on the ground: flat feet for CP and cavus feet in CMT. However, it should be noted that this work has certain limitations relating to the number of patients assessed, even though DMD and CMT diseases can be classed as rare diseases, qualifying this work as an exploratory study. Overall, our study suggests that the pelvis and the foot attitude play a crucial role in determining the biomechanical configuration for all four groups analyzed. Physical orthopaedic and rehabilitative treatment should consider the personal biomechanical configuration in terms of constrains of the gait. Each group showed a particular solution for balancing body segments, exploiting the available residual resources of the organism, both peripheral and central. Meanwhile, the spatial orientation of the thigh and leg were linked together in a sort of biomechanical invariance independent of the studied pathology. Both elements seem to contribute to a body configuration compatible with bipedal gait.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by “Bambino Gesù” Children’s Hospital. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author Contributions
SM, MF, AR, AP, SS, EC, and MP managed the overall project (conceptualization, methodology, and interpretation). SM and MP performed the data preprocessing, statistical analysis, results interpretation, and prepared the original manuscript draft. AP, MF, and AR performed the Gait Analysis data acquisition and database maintenance. GV and TS were responsible for the recruitment and clinical examinations and results interpretation. All authors critically reviewed, read, and approved the submitted version of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abu-Faraj, Z. O., Harris, G. F., Smith, P. A., and Hassani, S. (2015). “Human gait and clinical movement analysis,” in Wiley Encyclopedia of Electrical and Electronics Engineering, ed. J. G. Webster (New York, NY: John Wiley & Sons, Inc.).
Amori, V., Petrarca, M., Patané, F., Castelli, E., and Cappa, P. (2015). Upper body balance control strategy during continuous 3D postural perturbation in young adults. Gait Posture 41, 19–25. doi: 10.1016/j.gaitpost.2014.08.003
Armand, S., Decoulon, G., and Bonnefoy-Mazure, A. (2016). Gait analysis in children with cerebral palsy. EFORT Open Rev. 1, 448–460. doi: 10.1302/2058-5241.1.000052
Borghese, N. A., Bianchi, L., and Lacquaniti, F. (1996). Kinematic deferminants of human locomotion. J. Physiol. 494, 863–879. doi: 10.1113/jphysiol.1996.sp021539
Chruscikowski, E., Fry, N. R. D., Noble, J. J., Gough, M., and Shortland, A. P. (2017). Selective motor control correlates with gait abnormality in children with cerebral palsy. Gait Posture 52, 107–109. doi: 10.1016/j.gaitpost.2016.11.031
Cioni, G., Lodesani, M., Pascale, R., Coluccini, M., Sassi, S., Paolicelli, P. B., et al. (2008). The term diplegia should be enhanced. Part II: contribution to validation of the new rehabilitation oriented classification. Eur. J. Phys. Rehabil. Med. 44, 203–211.
Collins, S., Ruina, A., Tedrake, R., and Wisse, M. (2005). Efficient bipedal robots based on passive-dynamic walkers. Science 307, 1082–1085. doi: 10.1126/science.1107799
Crenna, P. (1998). Spasticity and “spastic” gait in children with cerebral palsy. Neurosci. Biobehav. Rev. 22, 571–578. doi: 10.1016/S0149-7634(97)00046-8
Davids, J. R., Cung, N. Q., Pomeroy, R., Schultz, B., Torburn, L., Kulkarni, V. A., et al. (2018). Quantitative Assessment of Knee Progression Angle during Gait in Children with Cerebral Palsy. J. Pediatr. Orthop. 38, e219–e224. doi: 10.1097/BPO.0000000000001134
Davies, B. T. (1968). A review of “ The Co-ordination and Regulation of Movements ” By N. Bernstein. (Pergamon Press, 1967.) [Pp. xii + 196.] 505. Ergonomics 11, 95–97. doi: 10.1080/00140136808930945
Doglio, L., Pavan, E., Pernigotti, I., Petralia, P., Frigo, C., and Minetti, C. (2011). Early signs of gait deviation in Duchenne muscular dystrophy. Eur. J. Phys. Rehabil. Med. 47, 587–594. doi: 10.1016/j.gaitpost.2008.10.044
Fonseca, S. T., Holt, K. G., Saltzman, E., and Fetters, L. (2001). A dynamical model of locomotion in spastic hemiplegic cerebral palsy: influence of walking speed. Clin. Biomech. 16, 793–805. doi: 10.1016/S0268-0033(01)00067-5
Gazzellini, S., Lispi, M. L., Castelli, E., Trombetti, A., Carniel, S., Vasco, G., et al. (2016). The impact of vision on the dynamic characteristics of the gait: strategies in children with blindness. Exp. Brain Res. 234, 2619–2627. doi: 10.1007/s00221-016-4666-9
Gibson, J. J. (1979). Gibson, James J. “The Theory of Affordances” The Ecological Approach to Visual Perception. Boston: Houghton Mifflin.
Goudriaan, M., Van den Hauwe, M., Dekeerle, J., Verhelst, L., Molenaers, G., Goemans, N., et al. (2018). Gait deviations in Duchenne muscular dystrophy—Part 1. A systematic review. Gait Posture 62, 247–261. doi: 10.1016/j.gaitpost.2018.03.020
Hamill, J., Knutzen, K. M., and Derrick, T. R. (2014). Biomechanical Basis of Human Movement, Fourth Edn. Philadelphia: Wolters Kluwer Health.
Holt, K. G., Fonseca, S. T., and Lafiandra, M. E. (2000). The dynamics of gait in children with spastic hemiplegic cerebral palsy: theoretical and clinical implications. Hum. Mov. Sci. 19, 375–405. doi: 10.1016/S0167-9457(00)00019-1
Kuniyoshi, Y., and Suzuki, S. (2004). “Dynamic emergence and adaptation of behavior through embodiment as coupled chaotic field,” in 2004 IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS), (Piscataway: IEEE).
Lacquaniti, F., Grasso, R., and Zago, M. (1999). Motor patterns in walking. News Physiol. Sci. 14, 168–174. doi: 10.1007/s11033-020-05699-710.1152/physiologyonline.1999.14.4.168
Lencioni, T., Piscosquito, G., Rabuffetti, M., Bovi, G., Di Sipio, E., Diverio, M., et al. (2017). Responsiveness of gait analysis parameters in a cohort of 71 CMT subjects. Neuromuscul. Disord. 27, 1029–1037. doi: 10.1016/j.nmd.2017.07.003
Rodda, J. M., Graham, H. K., Carson, L., Galea, M. P., and Wolfe, R. (2004). Sagittal gait patterns in spastic diplegia. J. Bone Jt. Surg. 86, 251–258. doi: 10.1302/0301-620X.86B2.13878
Romano, A., Favetta, M., Schirinzi, T., Summa, S., Minosse, S., D’Amico, A., et al. (2019). Evaluation of gait in Duchenne Muscular Dystrophy: relation of 3D gait analysis to clinical assessment. Neuromuscul. Disord. 29, 920–929. doi: 10.1016/j.nmd.2019.10.007
Romkes, J., and Brunner, R. (2007). An electromyographic analysis of obligatory (hemiplegic cerebral palsy) and voluntary (normal) unilateral toe-walking. Gait Posture 26, 577–586. doi: 10.1016/j.gaitpost.2006.12.010
Salami, F., Niklasch, M., Krautwurst, B. K., Dreher, T., and Wolf, S. I. (2017). What is the price for the Duchenne gait pattern in patients with cerebral palsy? Gait Posture 58, 453–456. doi: 10.1016/j.gaitpost.2017.09.006
Sienko Thomas, S., Buckon, C. E., Nicorici, A., Bagley, A., McDonald, C. M., and Sussman, M. D. (2010). Classification of the gait patterns of boys with Duchenne muscular dystrophy and their relationship to function. J. Child Neurol. 25, 1103–1109. doi: 10.1177/0883073810371002
Keywords: gait, cerebral palsy, Charcot-Marie-Tooth, Duchenne Muscular Dystrophy, absolute angle, biomechanical constraints
Citation: Minosse S, Favetta M, Romano A, Pisano A, Summa S, Schirinzi T, Vasco G, Castelli E and Petrarca M (2022) Comparison of the Gait Biomechanical Constraints in Three Different Type of Neuromotor Damages. Front. Hum. Neurosci. 16:822205. doi: 10.3389/fnhum.2022.822205
Received: 25 November 2021; Accepted: 28 February 2022;
Published: 29 March 2022.
Edited by:
Marco Iosa, Sapienza University of Rome, ItalyReviewed by:
Corrado Italo Angelini, University of Padua, ItalyRuxu Zhang, Central South University, China
Copyright © 2022 Minosse, Favetta, Romano, Pisano, Summa, Schirinzi, Vasco, Castelli and Petrarca. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Susanna Summa, susanna.summa@opbg.net
 Silvia Minosse
Silvia Minosse Martina Favetta
Martina Favetta Alberto Romano
Alberto Romano Alessandra Pisano1
Alessandra Pisano1  Susanna Summa
Susanna Summa Tommaso Schirinzi
Tommaso Schirinzi Maurizio Petrarca
Maurizio Petrarca