Abstract
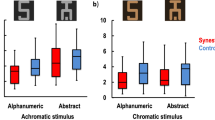
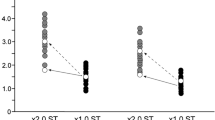
Diffusion tensor imaging allowed us to validate for the first time the hypothesis that hyperconnectivity causes the added sensations in synesthesia. Grapheme-color synesthetes (n = 18), who experience specific colors with particular letters or numbers (for example, 'R is sky blue'), showed greater anisotropic diffusion compared with matched controls. Greater anisotropic diffusion indicates more coherent white matter. Anisotropy furthermore differentiated subtypes of grapheme-color synesthesia. Greater connectivity in the inferior temporal cortex was particularly strong for synesthetes who see synesthetic color in the outside world ('projectors') as compared with synesthetes who see the color in their 'mind's eye' only ('associators'). In contrast, greater connectivity (as compared with non-synesthetes) in the superior parietal or frontal cortex did not differentiate between subtypes of synesthesia. In conclusion, we found evidence that increased structural connectivity is associated with the presence of grapheme-color synesthesia, and has a role in the subjective nature of synesthetic color experience.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Baron-Cohen, S., Burt, L., Smith-Laittan, F., Harrison, J. & Bolton, P. Synaesthesia: prevalence and familiality. Perception 25, 1073–1079 (1996).
Day, S.A. Some demographic and socio-cultural aspects of synesthesia. In Synesthesia: Perspectives from Cognitive Neuroscience (eds. Robertson, L. & Sagiv, N.) Ch. 2, 11–33 (Oxford University Press, Oxford, 2005).
Hubbard, E.M. & Ramachandran, V.S. Neurocognitive mechanisms of synesthesia. Neuron 48, 509–520 (2005).
Ramachandran, V.S. & Hubbard, E.M. Psychophysical investigations into the neural basis of synaesthesia. Proc. R. Soc. Lond. B 268, 979–983 (2001).
Mondloch, C.J. & Maurer, D. Do small white balls squeak? Pitch-object correspondences in young children. Cogn. Affect. Behav. Neurosci. 4, 133–136 (2004).
Hubbard, E.M., Arman, A.C., Ramachandran, V.S. & Boynton, G.M. Individual differences among grapheme-color synesthetes: brain-behavior correlations. Neuron 45, 975–985 (2005).
Ramachandran, V.S. & Hubbard, E.M. Synaesthesia: a window into perception, thought and language. J. Conscious. Stud. 8, 3–34 (2001).
Smilek, D., Dixon, M.J., Cuday, C. & Merikle, P.M. Synaesthetic photisms influence visual perception. J. Cogn. Neurosci. 13, 930–936 (2001).
Grossenbacher, P.G. & Lovelace, C.T. Mechanisms of synesthesia: cognitive and physiological constraints. Trends Cogn. Sci. 5, 36–41 (2001).
Esterman, M., Verstynen, T., Ivry, R.B. & Robertson, L.C. Coming unbound: disrupting automatic integration of synesthetic color and graphemes by transcranial magnetic stimulation of the right parietal lobe. J. Cogn. Neurosci. 18, 1570–1576 (2006).
Weiss, P.H., Zilles, K. & Fink, G.R. When visual perception causes feeling: enhanced cross-modal processing grapheme-color synesthesia. Neuroimage 28, 859–868 (2005).
Basser, P.J., Mattiello, J. & LeBihan, D. Estimation of the effective self-diffusion tensor from the NMR spin echo. J. Magn. Reson. B. 103, 247–254 (1994).
Basser, P.J. Inferring microstructural features and the physiological state of tissues from diffusion-weighted images. NMR Biomed. 8, 333–344 (1995).
Ward, J. & Mattingley, J. (eds.) Cognitive neuroscience perspectives on synaesthesia. Cortex 42 (special issue) (2006).
Dixon, M.J. & Smilek, D. The importance of individual differences in grapheme-color synesthesia. Neuron 45, 821–823 (2005).
Dixon, M.J., Smilek, D. & Merikle, P.M. Not all synaesthetes are created equal: projector versus associator synaesthetes. Cogn. Affect. Behav. Neurosci 4, 335–343 (2004).
Ishai, A., Ungerleider, L.G., Martin, A., Schouten, J.L. & Haxby, J.V. Distributed representation of objects in the human ventral visual pathway. Proc. Natl. Acad. Sci. USA 96, 9379–9384 (1999).
Martin, A., Wiggs, C.L., Ungerleider, L.G. & Haxby, J.V. Neural correlates of category-specific knowledge. Nature 379, 649–652 (1996).
Kanwisher, N., McDermott, J. & Chun, M.M. The fusiform face area: a module in human extrastriate cortex specialized for face perception. J. Neurosci. 17, 4302–4311 (1997).
Cohen, L. et al. The visual word form area: spatial and temporal characterization of an initial stage of reading in normal subjects and posterior split-brain patients. Brain 123, 291–307 (2000).
McKeefry, D.J. & Zeki, S. The position and topography of the human colour centre as revealed by functional magnetic resonance imaging. Brain 120, 2229–2242 (1997).
Smith, S.M. et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage 31, 1487–1505 (2006).
Nichols, T.E. & Holmes, A.P. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum. Brain Mapp. 15, 1–25 (2002).
Catani, M., Jones, D.K., Donato, R. & Ffytche, D.H. Occipito-temporal connections in the human brain. Brain 126, 2093–2107 (2003).
Evans, A.C.,, Collins, D.L. & Milner, B. An MRI-based stereotactic atlas from 250 young normal subjects. Soc. Neurosci. Abstr. 18, 408 (1992).
Duncan, R.O. & Boynton, G.M. Cortical magnification within human primary visual cortex correlates with acuity thresholds. Neuron 38, 659–671 (2003).
Nunn, J.A. et al. Functional magnetic resonance imaging of synesthesia: activation of V4/V8 by spoken words. Nat. Neurosci. 5, 371–375 (2002).
Sperling, J.M., Pruvolic, D., Linden, D.E.J., Singer, W. & Stirn, A. Neuronal correlates of colour-graphemic synaesthesia: a fMRI study. Cortex 42, 295–303 (2006).
Friedman-Hill, S.R., Robertson, L. & Treisman, A. Parietal contributions to visual feature binding: evidence from a patient with bilateral lesions. Science 269, 853–855 (1995).
Paulesu, E. et al. The physiology of coloured hearing. A PET activation study of colour-word synaesthesia. Brain 118, 661–676 (1995).
Muggleton, N., Tsakanikos, E., Walsh, V. & Ward, J. Disruption of synaesthesia following TMS of the right posterior parietal cortex. Neuropsychologia 45, 1582–1585 (2007).
Dehaene, S. & Naccache, L. Towards a cognitive neuroscience of consciousness: basic evidence and a workspace framework. Cognition 79, 1–37 (2001).
Dehaene, S. et al. Cerebral mechanisms of word masking and unconscious repetition priming. Nat. Neurosci. 4, 752–758 (2001).
Rees, G., Kreiman, G. & Koch, C. Neural correlates of consciousness in humans. Nat. Rev. Neurosci. 3, 261–270 (2002).
Lumer, E.D., Friston, K.J. & Rees, G. Neuronal correlates of perceptual rivalry in the human brain. Science 280, 1930–1934 (1998).
Lumer, E.D. & Rees, G.E. Covariation of activity in visual and prefrontal cortex associated with subjective visual perception. Proc. Natl. Acad. Sci. USA 96, 1669–1673 (1999).
Kleinschmidt, A., Buchel, C., Zeki, S. & Frackowiak, R.S.J. Human brain activity during spontaneously reversing perception of ambiguous figures. Proc. R. Soc. Lond. B 265, 2427–2433 (1998).
Smith, S.M. et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23, S208–S219 (2004).
Behrens, T.E.J. et al. Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magn. Reson. Med. 50, 1077–1088 (2003).
Smith, S.M. Fast robust automated brain extraction. Hum. Brain Mapp. 17, 143–155 (2002).
Rueckert, D. et al. Non-rigid registration using free-form deformations: application to breast MR images. IEEE Trans. Med. Imaging 18, 712–721 (1999).
Woolrich, M.W., Ripley, B.D., Brady, J.M. & Smith, S.M. Temporal autocorrelation in univariate linear modelling of FMRI data. Neuroimage 14, 1370–1386 (2001).
Beckmann, C.F., Jenkinson, M. & Smith, S.M. General multi-level linear modelling for group analysis in FMRI. Neuroimage 20, 1052–1063 (2003).
Woolrich, M.W., Behrens, T.E.J., Beckmann, C.F., Jenkinson, M. & Smith, S.M. Multi-level linear modelling for FMRI group analysis using Bayesian inference. Neuroimage 21, 1732–1747 (2004).
Worsley, K.J., Marrett, S., Neelin, P. & Evans, A.C. A three-dimensional statistical analysis for CBF activation studies in human brain. J. Cereb. Blood Flow Metab. 12, 900–918 (1992).
Acknowledgements
We thank I. Veer, M. Hillen, L. Zil, N. Rusiyanadi, F. van Klaveren and F. Binkhorst for their contribution to testing; all the synesthetic and control subjects for their enthusiastic collaboration; and B. Laeng and V. Lamme for their comments.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Main directions of white matter tracts in synesthetes and controls. (PDF 230 kb)
Supplementary Fig. 2
White matter tracts in synesthetes and controls. (PDF 848 kb)
Supplementary Table 1
Correlation matrix (nonparametric) of projector-associator score, FA values in the four clusters differentiating synesthetes from non-synesthetes, and BOLD signal in the four areas responding to synesthetic color in synesthetes. (PDF 57 kb)
Supplementary Table 2
Correlations between FA in inferior temporal cluster and BOLD signal in the anterior cluster in temporal cortex responding to synesthetic color, for synesthetes and non-synesthetes separately. (PDF 58 kb)
Rights and permissions
About this article
Cite this article
Rouw, R., Scholte, H. Increased structural connectivity in grapheme-color synesthesia. Nat Neurosci 10, 792–797 (2007). https://doi.org/10.1038/nn1906
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn1906
This article is cited by
-
An Open Science MRI Database of over 100 Synaesthetic Brains and Accompanying Deep Phenotypic Information
Scientific Data (2023)
-
A single case neuroimaging study of tickertape synesthesia
Scientific Reports (2023)
-
Synesthesia has specific cognitive processing during Go/No-go paradigms
Scientific Reports (2023)
-
An evolutionary account of impairment of self in cognitive disorders
Cognitive Processing (2023)
-
A case of co-occuring synesthesia, autism, prodigious talent and strong structural brain connectivity
BMC Psychiatry (2020)