Abstract
The leading hypothesis concerning the “reuse” or “recycling” of neural circuits builds on the assumption that evolution might prefer the redeployment of established circuits over the development of new ones. What conception of cognitive architecture can survive the evidence for this hypothesis? In particular, what sorts of “modules” are compatible with this evidence? I argue that the only likely candidates will, in effect, be the columns which Vernon Mountcastle originally hypothesized some 60 years ago, and which form part of the well-known columnar hypothesis in neuroscience—systems that cannot handle gross cognitive functions (vision, olfaction, language, etc.) as distinct from strictly exiguous subfunctions (such as aspects of edge detection, depth discrimination, etc.). This is in stark contrast to the modules postulated by much of cognitive psychology, cognitive neuropsychology, and evolutionary psychology. And yet the fate of this revised notion is unclear. The main issue confronting it is the effect of the neural network context on local function. At some point the effects of context are so strong that the degree of specialization required for modularity is not able to be met. Still, despite indications from neuroimaging that peripheral and central systems deploy shared circuitry, some skills clearly do seem to display modularization and autonomy. This article: (1) provides an in-depth analytical and historical review of the fortunes of modular thinking in cognitive science; (2) offers a systematic calibration of brain regions in terms of degrees of functional specificity and robustness; and (3) suggests another way of accounting for the partially encapsulated character of expertise and other highly practiced skills without having to resort to domain-specific modules.

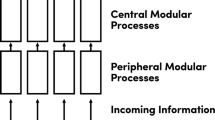
(Reproduced with permission from Anderson 2010, p. 247; figure copyright Cambridge University Press)
Similar content being viewed by others
Notes
Arguably Chomsky no longer holds to functional specialization in the sense of separate modifiability (see Zerilli 2017), but some passages in recent work suggest otherwise, e.g., intimating that Merge (the fundamental operation postulated within the so-called “Minimalist Program” in linguistics) is a self-contained generative device that dissociates from core motor operations (Berwick and Chomsky 2016, pp. 75–77).
“[Functional specialization] furnishes a kind of cognitive movable type for the mind, and mechanisms that can support robust laws, generalizations, and predictions (e.g., ‘forward’ inferences from cognitive tasks to brain areas) (Burnston 2016). If, for a given neural area A, there is some univocal description D such that D explains the functional role of A’s activity whenever A functions, it should be possible to formulate a theory tokening A providing ‘functional descriptions that apply over a range of instances of functioning,’ and ‘functional explanations in particular contexts that are relevant to contexts not yet explored’ (Burnston 2016, pp. 529, 531). This would be a ‘very powerful theory in terms of generalizability and projectability’ (Burnston 2016, p. 531)” (Zerilli 2017, p. 239).
This usage of “exaptation” is somewhat misleading, since exaptation usually implies loss of original function (see Godfrey-Smith 2001).
Of course what corresponds to a node in network neuroscience is somewhat arbitrary. For certain purposes neuroscientists may use a neuroanatomically delimited region as a node, whereas for other purposes they may use another one. I certainly would not wish to say that a module is something that depends on the interest of the neuroscientist. Additionally, the notion of a “module” in network neuroscience is different from the notion of a “node.” A module in network theory refers to a community of nodes (Zerilli 2017).
I hasten to add, however, that Anderson’s (2014) “dispositional vector” account of brain regions is an alternative strategy for coming to grips with the same set of issues. Others are clearly alive to the problem. Proponents of the Leabra architecture, for instance, resist modularist terminology precisely because it “forces a binary distinction on what is fundamentally a continuum” (Petrov et al. 2010, p. 287). See also Frost et al. (2015).
The scaling problem arises from the fact that as the number of neurons increases, the number of neurons that must be connected grows quadratically larger. As Gilbert (2013, p. 570) explains, “The clustering of neurons into functional groups, as in the columns of the cortex, allows the brain to minimize the number of neurons required for analyzing different attributes. If all neurons were tuned for every attribute, the resultant combinatorial explosion would require a prohibitive number of neurons.”
I am heavily indebted to Anderson (2014) for the review that follows.
References
Ackerman JM, Nocera CC, Bargh JA (2010) Incidental haptic sensations influence social judgments and decisions. Science 328:1712–1715
Altun ZF, Hall DH (2011) Nervous system, general description. In: Altun ZF, Herndon LA, Crocker C et al. (eds) WormAtlas http://www.wormatlas.org/ver1/handbook/contents.htm
Amaral DG, Strick PL (2013) The organization of the central nervous system. In: Kandel ER, Schwartz JH, Jessell TM et al (eds) Principles of neural science. McGraw-Hill, New York, pp 337–355
Anderson ML (2007a) Evolution of cognitive function via redeployment of brain areas. Neuroscientist 13:13–21
Anderson ML (2007b) Massive redeployment, exaptation, and the functional integration of cognitive operations. Synthese 159(3):329–345
Anderson ML (2007c) The massive redeployment hypothesis and the functional topography of the brain. Philos Psychol 21(2):143–174
Anderson ML (2008) Circuit sharing and the implementation of intelligent systems. Connect Sci 20(4):239–251
Anderson ML (2010) Neural reuse: a fundamental organizational principle of the brain. Behav Brain Sci 33(4):245–266; discussion 266–313. https://doi.org/10.1017/S0140525X10000853
Anderson ML (2014) After phrenology: neural reuse and the interactive brain. MIT Press, Cambridge
Anderson ML, Finlay BL (2014) Allocating structure to function: the strong links between neuroplasticity and natural selection. Front Hum Neurosci 7:1–16
Bach-y-Rita P (2004) Emerging concepts of brain function. J Integr Neurosci 4(2):183–205
Bargh JA, Williams LE, Huang JY et al (2010) From the physical to the psychological: mundane experiences influence social judgment and interpersonal behavior. Behav Brain Sci 33(4):267–268
Barrett HC (2006) Modularity and design reincarnation. In: Carruthers P, Laurence S, Stich SP (eds) The innate mind volume 2: culture and cognition. Oxford University Press, New York, pp 199–217
Barrett HC, Kurzban R (2006) Modularity in cognition: framing the debate. Psychol Rev 113(3):628–647
Bechtel W (2008) Mental mechanisms: philosophical perspectives on cognitive neuroscience. Routledge, London
Bergeron V (2007) Anatomical and functional modularity in cognitive science: shifting the focus. Philos Psychol 20(2):175–195
Berwick RC, Chomsky N (2016) Why only us: language and evolution. MIT Press, Cambridge
Binkofski F, Amunts K, Stephan KM et al (2000) Broca’s region subserves imagery of motion: a combined cytoarchitectonic and fMRI study. Hum Brain Mapp 11:273–285
Bressler SL (1995) Large-scale cortical networks and cognition. Brain Res Rev 20:288–304
Burnston DC (2016) A contextualist approach to functional localization in the brain. Biol Philos 31:527–550
Buxhoeveden DP, Casanova MF (2002) The minicolumn hypothesis in neuroscience. Brain 125:935–951
Carruthers P (2006) The architecture of the mind: massive modularity and the flexibility of thought. Oxford University Press, Oxford
Carruthers P (2008) Précis of the architecture of the mind: massive modularity and the flexibility of thought. Mind Lang 23(3):257–262
Casasanto D, Dijkstra K (2010) Motor action and emotional memory. Cognition 115(1):179–185
Chomsky N (1980) Rules and representations. Columbia University Press, New York
Chomsky N (1988) Language and problems of knowledge: the managua lectures. MIT Press, Cambridge
Chomsky N (2002) On nature and language. Cambridge University Press, New York
Coase RH (1937) The nature of the firm. Economica New Series 4(16):386–405
Cole MW, Reynolds JR, Power JD et al (2013) Multi-task connectivity reveals flexible hubs for adaptive task control. Nat Neurosci 16(9):1348–1355
Coltheart M (2011) Methods for modular modelling: additive factors and cognitive neuropsychology. Cogn Neuropsychol 28(3–4):224–240
Cosmides L, Tooby J (1994) Origins of domain specificity: the evolution of functional organization. In: Hirschfield L, Gelman S (eds) Mapping the world: domain specificity in cognition and culture. Cambridge University Press, New York, pp 85–116
Craver CF (2007) Explaining the brain. Oxford University Press, Oxford
da Costa NM, Martin KAC (2010) Whose cortical column would that be? Front Neuroanat 4(5):1–10
Damasio AR, Tranel D (1993) Nouns and verbs are retrieved with differently distributed neural systems. Proc Natl Acad Sci USA 90:4957–4960
Damasio H, Grabowski TJ, Tranel D et al (1996) A neural basis for lexical retrieval. Science 380:499–505
Deacon TW (2010) A role for relaxed selection in the evolution of the language capacity. Proc Natl Acad Sci USA 107:9000–9006
Decety J, Grèzes J, Costes N et al (1997) Brain activity during observation of actions. Influence of action content and subject’s strategy. Brain 120(10):1763–1777
Dehaene S (2005) Evolution of human cortical circuits for reading and arithmetic: the “neuronal recycling” hypothesis. In: Dehaene S, Duhamel JR, Hauser MD, Rizzolatti G (eds) From monkey brain to human brain. MIT Press, Cambridge, pp 133–157
Dehaene S, Bossini S, Giraux P (1993) The mental representation of parity and numerical magnitude. J Exp Psychol Gen 122:371–396
Edelman GM, Gally JA (2001) Degeneracy and complexity in biological systems. Proc Natl Acad Sci USA 98(24):13763–13768
Fedorenko E, Thompson-Schill SL (2014) Reworking the language network. Trends Cogn Sci 18(3):120–126
Fedorenko E, Behr MK, Kanwisher N (2011) Functional specificity for high-level linguistic processing in the human brain. Proc Natl Acad Sci USA 108(39):16428–16433
Fodor JA (1983) The modularity of mind: an essay on faculty psychology. MIT Press, Cambridge
Frost R, Armstrong BC, Siegelman N, Christiansen MH (2015) Domain generality versus modality specificity: the paradox of statistical learning. Trends Cogn Sci 19(3):117–125
Gall FJ, Spurzheim JC (1835) On the function of the brain and each of its parts. Marsh Capen and Lyon, Boston
Gauthier I, Curran T, Curby KM, Collins D (2003) Perceptual interference supports a non-modular account of face processing. Nat Neurosci 6(4):428–432
Gazzaniga MS (1989) Organization of the human brain. Science 245(4921):947–952
Gilbert CD (2013) The constructive nature of visual processing. In: Kandel ER, Schwartz JH, Jessell TM et al (eds) Principles of neural science. McGraw-Hill, New York, pp 556–576
Glenberg AM, Kaschak MP (2002) Grounding language in action. Psychon Bull Rev 9:558–565
Glenberg AM, Brown M, Levin JR (2007) Enhancing comprehension in small reading groups using a manipulation strategy. Contemp Educ Psychol 32:389–399
Glenberg AM, Sato M, Cattaneo L (2008) Use-induced motor plasticity affects the processing of abstract and concrete language. Curr Biol 18(7):R290–R291
Godfrey-Smith P (2001) Three kinds of adaptationism. In: Orzack SH, Sober E (eds) Adaptationism and optimality. Cambridge University Press, Cambridge, pp 335–357
Gold I, Roskies AL (2008) Philosophy of neuroscience. In: Ruse M (ed) The oxford handbook of philosophy of biology. Oxford University Press, New York, pp 349–380
Graziano MSA, Taylor CSR, Moore T, Cooke DF (2002) The cortical control of movement revisited. Neuron 36:349–362
Guida A, Campitelli G, Gobet F (2016) Becoming an expert: ontogeny of expertise as an example of neural reuse. Behav Brain Sci 39:13–15
Hubbard EM, Piazza M, Pinel P, Dehaene S (2005) Interactions between number and space in parietal cortex. Nat Rev Neurosci 6(6):435–448
Iriki A, Taoka M (2012) Triadic (ecological, neural, cognitive) niche construction: a scenario of human brain evolution extrapolating tool use and language from the control of reaching actions. Philos Trans R Soc B 367:10–23
Jacobs JA (1999) Computational studies of the development of functionally specialized neural modules. Trends Cogn Sci 3(1):31–38
Jungé JA, Dennett DC (2010) Multi-use and constraints from original use. Behav Brain Sci 33(4):277–278
Kaan E, Stowe LA (2002) Storage and computation in the brain: A neuroimaging perspective. In: Nooteboom SG, Weerman F, Wijnen FNK (eds) Storage and computation in the language faculty. Kluwer, Dordrecht, pp 257–298
Kandel ER, Hudspeth AJ (2013) The brain and behavior. In: Kandel ER, Schwartz JH, Jessell TM et al (eds) Principles of neural science. McGraw-Hill, New York, pp 5–20
Karmiloff-Smith A (1992) Beyond modularity: a developmental perspective on cognitive science. MIT Press, Cambridge
Klein C (2010) Redeployed functions versus spreading activation: a potential confound. Behav Brain Sci 33(4):280–281
Klein C (2012) Cognitive ontology and region-versus network-oriented analyses. Philos Sci 79(5):952–960
Krubitzer L (1995) The organization of neocortex in mammals: are species differences really so different? Trends Neurosci 18(9):408–417
Laurence S, Margolis E (2015) Concept nativism and neural plasticity. In: Margolis E, Laurence S (eds) The conceptual mind: new directions in the study of concepts. MIT Press, Cambridge, pp 117–147
Leise EM (1990) Modular construction of nervous systems: a basic principle of design for invertebrates and vertebrates. Brain Res Rev 15:1–23
MacNeilage PF (1998) The frame/content theory of evolution of speech production. Behav Brain Sci 21(4):499–511. discussion 511–546
Maess B, Koelsch S, Gunter TC, Friederici AD (2001) Musical syntax is processed in Broca’s area: an MEG study. Nat Neurosci 4:540–545
Maleszka R, Mason PH, Barron AB (2013) Epigenomics and the concept of degeneracy in biological systems. Briefings Funct Genomics 13(3):191–202
Marr D (1976) Early processing of visual information. Philos Trans R Soc B 275:483–524
Martin A, Haxby JV, Lalonde FM, Wiggs CL, Ungerleider LG (1995) Discrete cortical regions associated with knowledge of color and knowledge of action. Sci 270:102–105
Martin A, Wiggs CL, Ungerleider LG, Haxby JV (1996) Neural correlates of category-specific knowledge. Nature 379(6566):649–652
Martin A, Ungerleider LG, Haxby JV (2000) Category-specificity and the brain: the sensorymotor model of semantic representations of objects. In: Gazzaniga MS (ed) The new cognitive neurosciences, 2nd edn. MIT Press, Cambridge, pp 1023–1036
Mason PH (2010) Degeneracy at multiple levels of complexity. Biol Theory 5(3):277–288
Mather M, Cacioppo JT, Kanwisher N (2013) How fMRI can inform cognitive theories. Perspect Psychol Sci 8(1):108–113
Mountcastle VB (1957) Modality and topographic properties of single neurons of cat’s somatic sensory cortex. J Neurophysiol 20(4):408–434
Mountcastle VB (1978) An organizing principle for cerebral function: the unit module and the distributed system. In: Edelman G, Mountcastle VB (eds) The mindful brain. MIT Press, Cambridge, pp 7–50
Mountcastle VB (1997) The columnar organization of the neocortex. Brain 120:701–722
Nishitani N, Schürmann M, Amunts K, Hari R (2005) Broca’s region: from action to language. Physiology 20:60–69
O’Reilly RC, Munakata Y, Frank MJ et al (2012) Computational cognitive neuroscience. 1st edn. Wiki Book. http://ccnbook.colorado.edu
Ohlsson S (1994) Representational change, generality versus specificity, and nature versus nurture: perennial issues in cognitive research. Behav Brain Sci 17(4):724–725
Parfit D (1984) Reasons and persons. Oxford University Press, Oxford
Pascual-Leone A, Hamilton R (2001) The metamodal organization of the brain. Prog Brain Res 134:427–445
Pascual-Leone A, Amedi A, Fregni F, Merabet LB (2005) The plastic human brain cortex. Annu Rev Neurosci 28:377–401
Pasqualotto A (2016) Multisensory integration substantiates distributed and overlapping neural networks. Behav Brain Sci 39:20–21
Pessoa L (2016) Beyond disjoint brain networks: overlapping networks for cognition and emotion. Behav Brain Sci 39:22–24
Petrov AA, Jilk DJ, O’Reilly RC (2010) The Leabra architecture: specialization without modularity. Behav Brain Sci 33(4):286–287
Poldrack RA (2010) Mapping mental function to brain structure: how can cognitive neuroimaging succeed? Perspect Psychol Sci 5(6):753–761
Poldrack RA, Halchenko YO, Hanson SJ (2009) Decoding the large-scale structure of brain function by classifying mental states across individuals. Psychol Sci 20(11):1364–1372
Price CJ, Friston KJ (2005) Functional ontologies for cognition: the systematic definition of structure and function. Cogn Neuropsychol 22(3):262–275
Prinz JJ (2006) Is the mind really modular? In: Stainton R (ed) Contemporary debates in cognitive science. Blackwell, Oxford, pp 22–36
Pulvermüller F (2005) Brain mechanisms linking language and action. Nat Rev Neurosci 6:576–582
Pulvermüller F, Fadiga L (2010) Active perception: sensorimotor circuits as a cortical basis for language. Nat Rev Neurosci 11:351–360
Rasmussen J (1986) Information processing and human-machine interaction. North-Holland, Amsterdam
Rockland KS (2010) Five points on columns. Front Neuroanat 4(6):1–10
Rowland DC, Moser MB (2014) From cortical modules to memories. Curr Opin Neurobiol 24:22–27
Sperber D (1994) The modularity of thought and the epidemiology of representations. In: Hirschfield LA, Gelman SA (eds) Mapping the mind. Cambridge University Press, Cambridge, pp 39–67
Sperber D (2002) In defense of massive modularity. In: Dupoux I (ed) Language, brain, and cognitive development. MIT Press, Cambridge, pp 47–57
Sporns O (2015) Network neuroscience. In: Marcus G, Freeman J (eds) The future of the brain. Princeton University Press, Princeton, pp 90–99
Stanton NA, Salmon PM (2009) Human error taxonomies applied to driving: a generic error taxonomy and its implications for intelligent transport systems. Saf Sci 47(2):227–237
Sternberg S (2011) Modular processes in mind and brain. Cogn Neuropsychol 28(3–4):156–208
Thoenissen D, Zilles K, Toni I (2002) Differential involvement of parietal and precentral regions in movement preparation and motor intention. J Neurosci 22:9024–9034
Walker GH, Stanton NA, Salmon PM (2015) Human factors in automotive engineering and technology. Ashgate, Surrey
Wernicke C (1908) The symptom-complex of aphasia. In: Church A (ed) Diseases of the nervous system. Appleton, New York, pp 265–324
Whiteacre JM (2010) Degeneracy: a link between evolvability, robustness and complexity in biological systems. Theor Biol Med Model 7(6):1–17
Williams LE, Bargh JA (2008a) Experiencing physical warmth promotes interpersonal warmth. Science 322:606–607
Williams LE, Bargh JA (2008b) Keeping one’s distance: the influence of spatial distance cues on affect and evaluation. Psychol Sci 19:302–308
Zador A (2015) The connectome as a DNA sequencing problem. In: Marcus G, Freeman J (eds) The future of the brain. Princeton University Press, Princeton, pp 40–49
Zerilli J (2017) Against the “system” module. Philos Psychol 30(3):235–250
Zerilli J (2019) Neural redundancy and its relation to neural reuse. Philos Sci (forthcoming)
Funding
This research was supported by an Australian Government Research Training Program Scholarship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zerilli, J. Neural Reuse and the Modularity of Mind: Where to Next for Modularity?. Biol Theory 14, 1–20 (2019). https://doi.org/10.1007/s13752-018-0309-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13752-018-0309-7