- 1School of Physical Education, Soochow University, Suzhou, China
- 2Department of Physical Education, Wuhan University of Technology, Wuhan, China
- 3College of Physical Education, Hubei University of Arts and Science, Xiangyang, China
Objectives: The present study aimed to systematically analyze the effects of physical activity on executive function, working memory, cognitive flexibility, and activities of daily living (ADLs) in Alzheimer's disease (AD) patients and to provide a scientific evidence-based exercise prescription.
Methods: Both Chinese and English databases (PubMed, Web of Science, the Cochrane Library, EMBASE, VIP Database for Chinese Technical Periodicals, China National Knowledge Infrastructure, and Wanfang) were used as sources of data to search for randomized controlled trials (RCTs) published between January 1980 and December 2019 relating to the effects of physical activity on executive function, working memory, cognitive flexibility, and ADL issues in AD patients. Sixteen eligible RCTs were ultimately included in the meta-analysis.
Results: Physical activity had significant benefits on executive function [standard mean difference (SMD) = 0.42, 95% confidence interval (CI) 0.22–0.62, p < 0.05], working memory (SMD = 0.28, 95% CI 0.11–0.45, p < 0.05), cognitive flexibility (SMD = 0.23, 95% CI −0.02 to 0.47, p < 0.01), and ADLs (SMD = 0.68, 95% CI 0.19–1.16, p < 0.05) among AD patients. Subgroup analysis indicated that, for executive function issues, more than 60 min per session for 16 weeks of moderate-to-high-intensity dual-task exercises or multimodal exercise had a greater effect on AD patients. For working memory and cognitive flexibility issues, 60–90 min of moderate-intensity dual-task exercises 1–4 times/week was more effective. For ADL issues, 30–90 min of multimodal exercise at 60–79% of maximal heart rate (MHR) 3–4 times/week had a greater effect on AD patients.
Conclusions: Physical activity was found to lead to significant improvements in executive function, working memory, cognitive flexibility, and ADLs in AD patients and can be used as an effective method for clinical exercise intervention in these patients. However, more objective, scientific, and effective RCTs are needed to confirm this conclusion.
Introduction
Alzheimer's disease (AD) is a common disorder of the nervous system, accounting for disease in 60–70% of patients with dementia (Reitz et al., 2011), causing severe clinical, social, and economic problems (Prince et al., 2013). Features of dementia include progressive cognitive decline, including loss of memory, language, or executive function, and subsequent decline of social function, for example, activities of daily living (ADLs) (Allal et al., 2015). The cognitive process of the frontal lobe may also change, which is characterized by decreased attention and executive function, as evidenced by deficits in problem solving, planning, and organizing behavior and ideas, abstraction, judgment, cognitive flexibility, decision making, working memory, and self-monitoring (Avilla and Miotto, 2002; Yaari and Bloom, 2007). By 2050, the number of people ≥60 years old will increase by 1.25 billion (Prince et al., 2013), and there will be an estimated 115.4 million people with dementia (Maffei et al., 2017). Drug therapy has been shown to be beneficial to the cognitive function and dependence in ADLs in AD patients (Tan et al., 2014), but it also has side effects.
Based on the above factors, alternative treatment options for AD are necessary to achieve better treatment results. Some research shows that approximately one-third of all AD cases may be due to potentially modifiable factors, such as lack of physical activity (Norton et al., 2014), which means that the disease can be prevented. Furthermore, some human and animal studies have shown that physical activity can promote the improvement of cerebrovascular function, perfusion, and brain neural plasticity, which can prevent the gradual loss of cognitive function or executive function related to diseases, such as aging and dementia (Davenport et al., 2012; Erickson et al., 2012). Furthermore, physical activity is considered to have a significant effect on executive function (Wilbur et al., 2012), as confirmed recently in a large experiment of moderate-intensity exercise in sedentary older adults: in the subgroup with the weakest cognitive ability, executive function was improved. In recent years, more and more studies have confirmed the positive effect of physical activity among AD patients. Meanwhile, executive function can directly affect the ADLs or continued independence (Royall et al., 2000; Bell-McGinty et al., 2002; Cahn-Weiner et al., 2002). Some studies provide support for the hypothesis that commonly used clinical trials of executive function significantly predict the ADLs (Bell-McGinty et al., 2002; Cahn-Weiner et al., 2002). As such, with a decrease in executive function, a breakdown in successful execution and completion of complex behavioral procedures is likely, especially in subsets of ADLs involving executive control (Bell-McGinty et al., 2002). However, some ADLs (e.g., ambulating, cooking, reading, leisure, housework, and managing finances) promote improved physical, cognitive, and executive functions (Bell-McGinty et al., 2002; Cahn-Weiner et al., 2002; Jekel et al., 2015). More and more studies have shown that physical activity has a significant impact on improving executive function and ADL issues in AD patients, thus improving their quality of life. In addition, the World Health Organization (WHO) recommends that people over the age of 65 should take at least 150 min of moderate-intensity aerobic exercise (such as brisk walking and jogging) every week, 75 min of high-intensity aerobic exercise every week, or a combination of the two supplemented by muscle-strengthening activities (such as resistance exercise and stretching exercise) on 2 or more days every week (World Health Organization Physical Activity Older Adults, 2017).
Recently, a great deal of research has been carried out to evaluate the impact of physical activity on executive function or ADL issues among AD patients. Because of the differences in the intervention samples, timing, frequency, intensity, and duration, the specific effects on executive function and ADL issues among AD patients could have been different. Therefore, the aim of our meta-analysis was to evaluate the impact of physical activity on executive function and ADL issues in AD patients. Moreover, executive function is a complex construct that includes different functions, such as cognitive flexibility inhibition and working memory. However, there are few studies on inhibitory functions in AD patients. Therefore, this study assessed the specific effects of physical activity on cognitive flexibility and working memory issues in AD patients. This study also explored the internal regulation mechanism of physical activity on the executive functions of AD patients to provide a corresponding exercise prescription.
Methods
Search Strategy
Literature was identified using the following databases: PubMed, Web of Science, the Cochrane Library, EMBASE, VIP Database for Chinese Technical Periodicals, China National Knowledge Infrastructure, and Wanfang. These databases were searched to identify randomized controlled trials (RCTs) published in any language between January 1, 1980 and December 31, 2019. The search terms used included “exercise or physical activity or aerobic exercise or physical exercise or aerobic fitness or walking or cycling or strength training or balance training or flexibility training” with AD terms including “AD or Alzheimer's disease or Alzheimer” as well as “executive function or executive functions or ADL or activities of daily living.”
Inclusion Criteria
The selection criteria were as follows: (1) RCTs investigating the impact of any type of physical activity as an additional intervention on executive function or ADLs; (2) sample population including a group of old people (aged ≥50 years) and participants diagnosed with Alzheimer's-type dementia of any severity, excluding diagnoses of other dementias or mild cognitive impairment (MCI); (3) interventions in an experimental group involving physical activity (e.g., aerobic exercise, aerobic fitness, walking, cycling, strength training, balance training, and flexibility training) compared with different types of control groups (e.g., usual care, no physical activity, and no-intervention control group); (4) outcome indicators including test data on executive function and ADLs; and (5) publication language of Chinese or English.
Exclusion Criteria
The exclusion criteria were as follows: (1) duplicated studies; (2) reviews, observational studies, abstract-only articles (without full-text article available), and non-RCT studies; and (3) studies with no data or unclear data reported for analysis.
Collection of Studies
Two investigators (LZ and LW) independently reviewed the titles and abstracts from the search results and screened out the full texts that might meet the criteria. If a study met the inclusion criteria, it received a full-text article evaluation. When there was any disagreement between the two reviewers, a third reviewer (LL) was invited to discuss with them and to verify the eligibility of the uncertain article. All eligible studies included information, such as author, publication year, country, sample size, sample population age, intervention methods, duration, measurement standards, experimental results, and dropouts.
Data Extraction
Detailed information included the first author, publication year, participant characteristics (sample size and age range/mean age), intervention design (frequency, duration of each intervention session, duration, and follow-up), outcome measure, statistical analyses, and results. Meanwhile, we also extracted quantitative data from the research results: mean and standard deviation (SD) of executive function and ADLs between physical activity and usual care, including its corresponding sample size.
Methodological Quality Assessment
Two authors used the modified the Physical Therapy Evidence Database (PEDro) scale (Zou et al., 2019) to independently perform methodological quality assessment of each eligible study. This assessment consisted of nine items (randomization, concealed allocation, similar baseline, blinding of assessors, ≤15% dropouts, intention-to-treat analysis, between-group comparison, point measure and measures of variability, and isolate exercise intervention), and higher scores indicate better quality of the method.
Statistical Analysis
Stata 14.0 (StataCorp, Texas, USA) was used to calculate effect sizes [standardized mean difference (SMD)] of physical activity on executive function and ADLs. SMD was considered as small (0.2–0.49), moderate (0.5–0.79), or large (0.8). According to the intervention system review of the Cochrane Collaboration handbook, selection of fixed-effects or random-effects meta-analysis should be based on the actual effect of an intervention on outcome measures. Differences (standard mean difference, SMD) and 95% confidence intervals (95% CIs) were calculated. I2 values of 25, 50, and 75% are considered to be low, medium, and high heterogeneity (Higgins et al., 2003). When the heterogeneity test I2 ≥ 50%, a random-effects model was used for meta-analysis. In this study, regression analysis was used to study the degree of experimental heterogeneity. Subgroup analyses were performed according to categorical variables, including sample age, exercise intensity, frequency, duration, duration of each intervention session, and exercise type. Subgroup analysis was used to determine which subgroup was more effective for improving the executive function and ADLs among AD patients.
Results
Study Selection
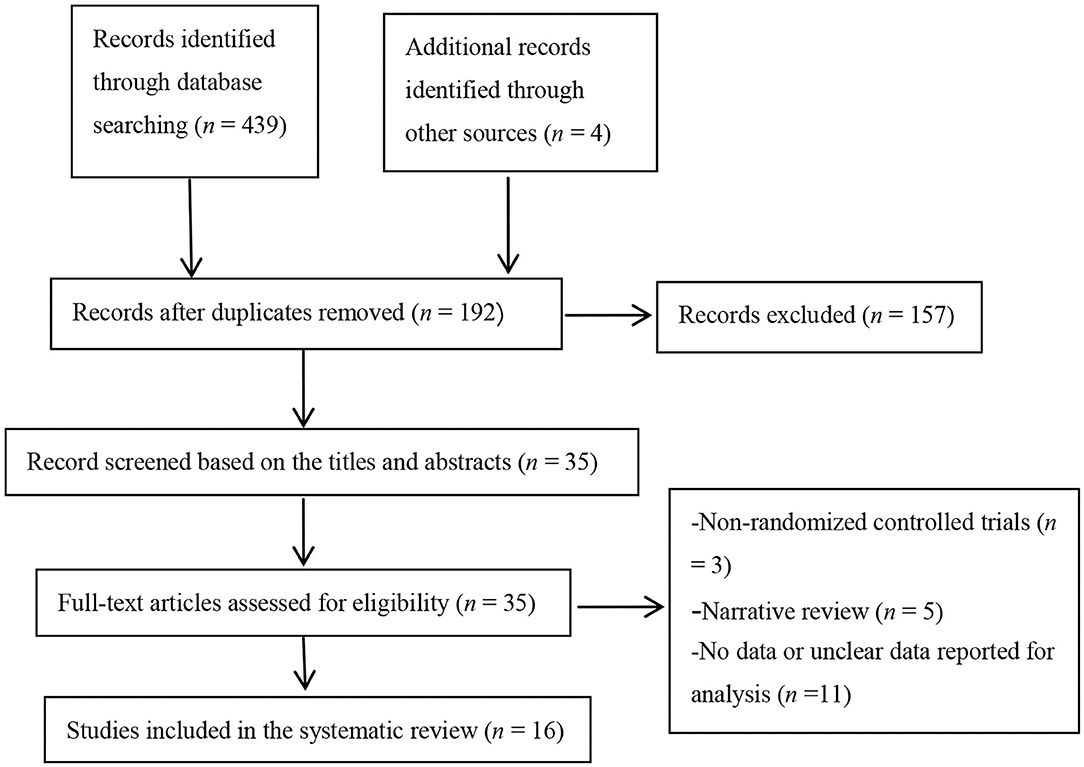
A total of 443 topic-related articles were identified from 7 databases and other resources (Figure 1). After removing duplicate articles, 192 articles remained. A total of 157 articles were deleted after titles and abstracts were screened for non-related articles (n = 148) and abstract-only articles (n = 9). The remaining 35 articles were further screened after reading the full-text articles. Nineteen studies were removed because they were non-RCTs (n = 3), reviews (n = 5), or had no or unclear outcome measures (n = 11). Finally, our meta-analysis included 16 eligible studies.
Characteristics of Eligible Studies
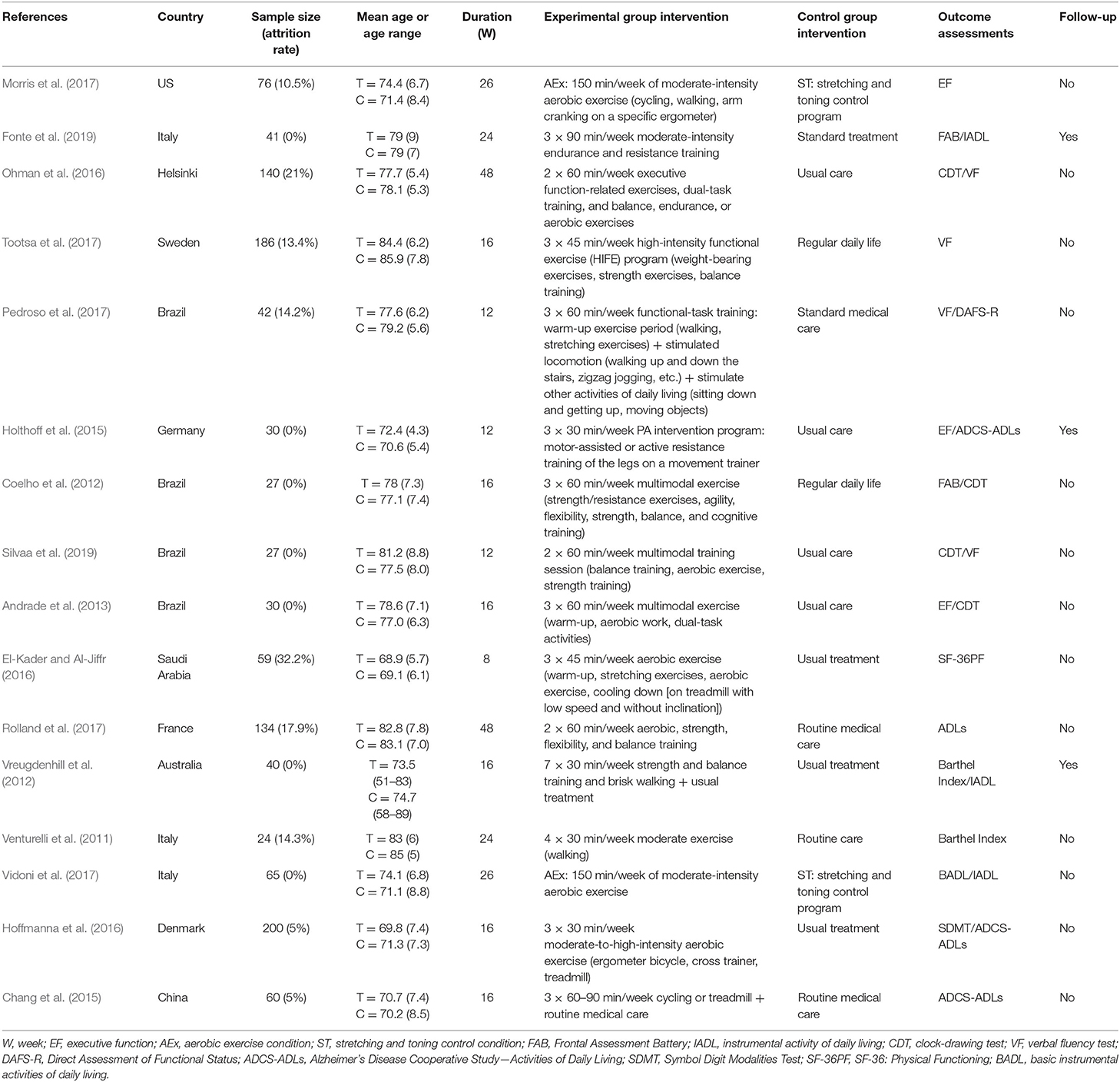
There were 16 eligible (Venturelli et al., 2011; Coelho et al., 2012; Vreugdenhill et al., 2012; Andrade et al., 2013; Chang et al., 2015; Holthoff et al., 2015; El-Kader and Al-Jiffr, 2016; Hoffmanna et al., 2016; Ohman et al., 2016; Morris et al., 2017; Pedroso et al., 2017; Rolland et al., 2017; Tootsa et al., 2017; Vidoni et al., 2017; Fonte et al., 2019; Silvaa et al., 2019) RCTs, as shown in Table 1. There were 1,181 participants in total; the smallest sample was 27 participants (Silvaa et al., 2019), and the largest sample was 200 participants (Holthoff et al., 2015). The age of the participants in the experiment ranged from 50 to 96 years old. The shortest experimental period was 8 weeks (El-Kader and Al-Jiffr, 2016), and the longest experimental period was 1 year (Rolland et al., 2017). The experimental group included various interventions, such as moderate-to-high-intensity physical activity, ergometer bicycle exercise, walking, cycling, strength training, balance training, and flexibility training. The control group was treated by usual care, standard treatment, routine medical care, etc. (Table 1).
Methodological Quality Assessment
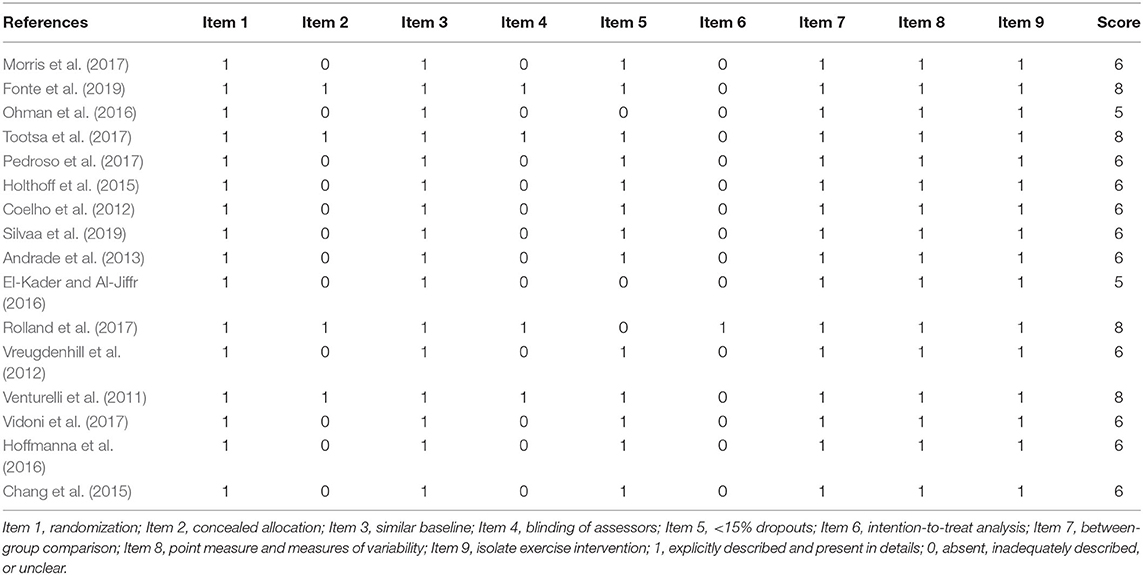
The methodological quality score for all qualified studies was between 5 and 8 (Table 2). All studies were RCTs and had similar baseline characteristics, between-group comparisons, point measures, measures of variability description, and isolated exercise interventions. Only four studies had concealed allocations and blinding of assessors (Venturelli et al., 2011; Rolland et al., 2017; Tootsa et al., 2017; Fonte et al., 2019). The dropout rates in three of these studies were all higher than 15% (El-Kader and Al-Jiffr, 2016; Ohman et al., 2016; Rolland et al., 2017), and only one study used the intention-to-treat principle (Rolland et al., 2017).
Meta-Analysis of Outcome Indicators
Effect of Physical Activity on Executive Function Issues in AD Patients
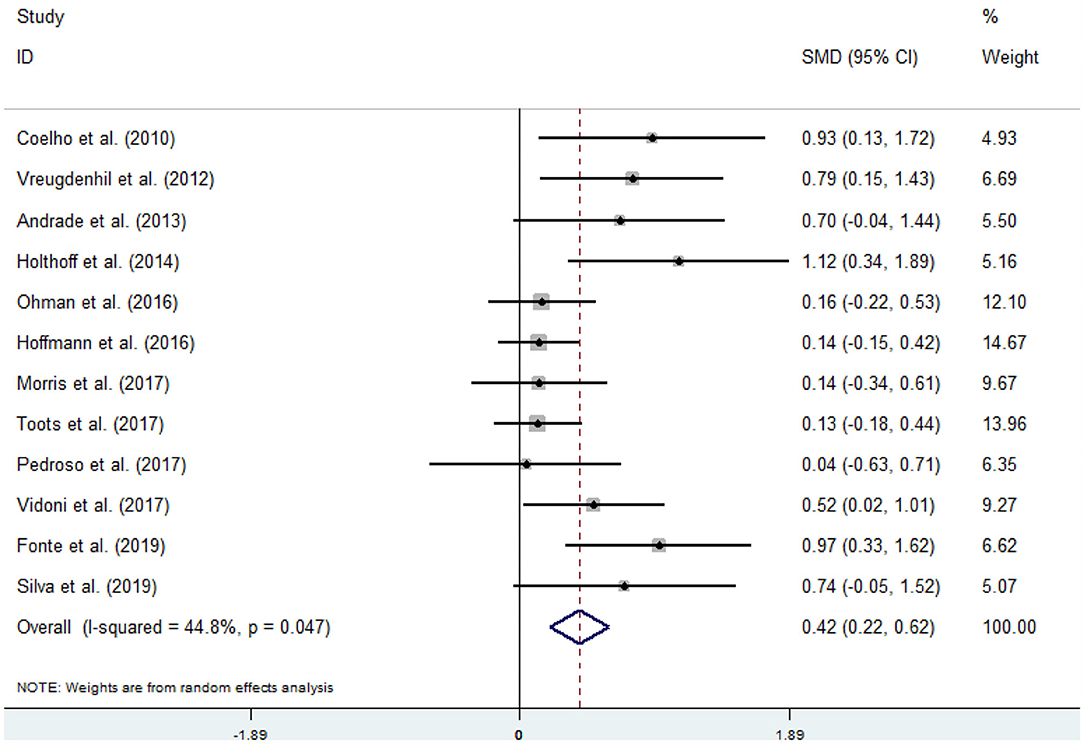
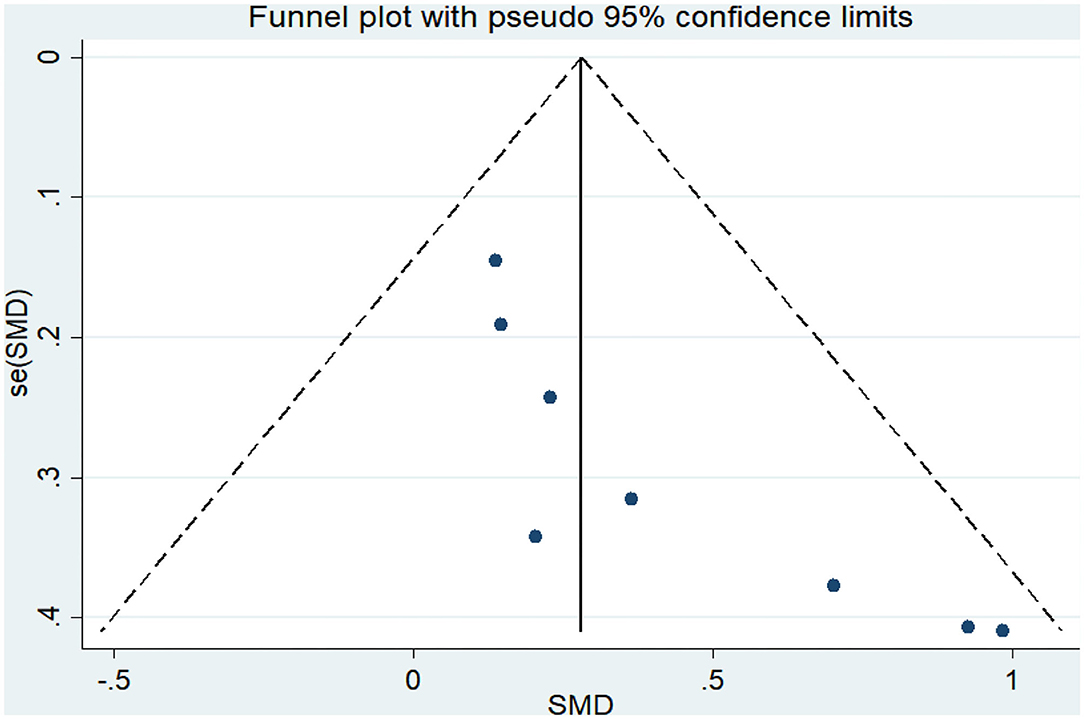
Twelve articles (Coelho et al., 2012; Vreugdenhill et al., 2012; Andrade et al., 2013; Holthoff et al., 2015; Hoffmanna et al., 2016; Ohman et al., 2016; Morris et al., 2017; Pedroso et al., 2017; Tootsa et al., 2017; Vidoni et al., 2017; Fonte et al., 2019; Silvaa et al., 2019) compared the effects of an intervention group and a control group on executive function before and after the experiment. An asymmetrical funnel plot was presented. The funnel plot shows that there were no outlier values (Figure 2). There was a moderate heterogeneity in the research literature (p = 0.047, I2 = 44.8%), and a random-effects model was selected for the meta-analysis (Figure 3). The meta-analysis of 12 studies demonstrated that physical activity had significant effects on improving executive function in AD patients (SMD = 0.42, 95% CI 0.22–0.62, p < 0.05).
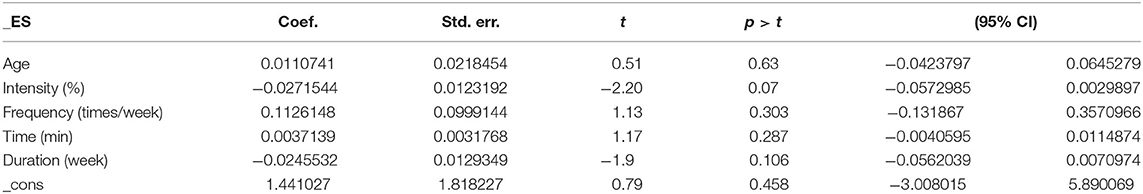
Covariates including age, intensity, frequency, time, and duration are likely to be the influencing factors for executive function issues in AD patients. The results of the regression of covariates for executive function issues in AD patients are presented in Table 3. For executive function, there were no significant effects of age (95% CI −0.0423797 to 0.0645279, p = 0.63), intensity (95% CI −0.0572985 to 0.0029897, p = 0.07), frequency (95% CI −0.131867 to 0.3570966, p = 0.303), time (95% CI −0.0040595 to 0.0114874, p = 0.287), or duration (95% CI −0.0562039 to 0.0070974, p = 0.106).
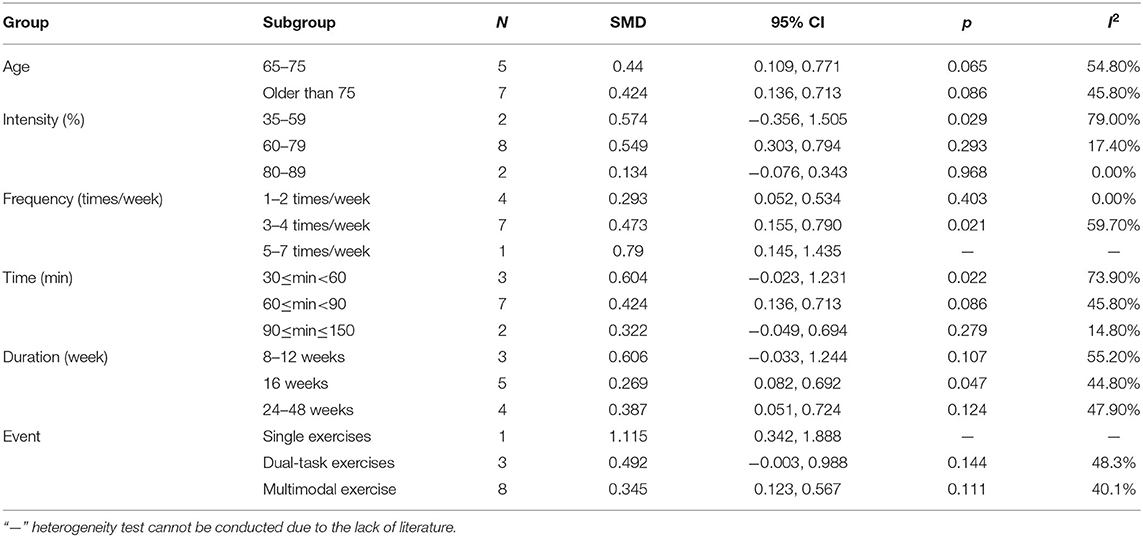
According to the sample population's age, exercise intensity, frequency, time, and duration, this study divided the research subjects into different subgroups, as shown in Table 4. The results from the subgroup analysis are as follows: (1) age: physical activity was beneficial for AD patients who were older than 75 years and had executive function issues. (2) Intensity: for executive function issues in AD patients, maintaining a 60–79 or an 80–89% maximal heart rate (MHR) during physical activity was more effective. (3) Frequency: the frequency of physical activity has a significant impact on the improvement of the executive function in AD patients, and the effect of 1–2 times/week was better than the effect of 3–4 or 5–7 times/week. (4) Time: a total of 60–150 min of physical activity per exercise session significantly improved executive function in AD patients. (5) Duration: an intervention duration of 16 or 24–48 weeks showed a significant effect on executive function issues in AD patients. (6) Event: for executive function issues in AD patients, both dual-task exercises and multimodal exercise had significant effects.
Effect of Physical Activity on Working Memory Issues in AD Patients
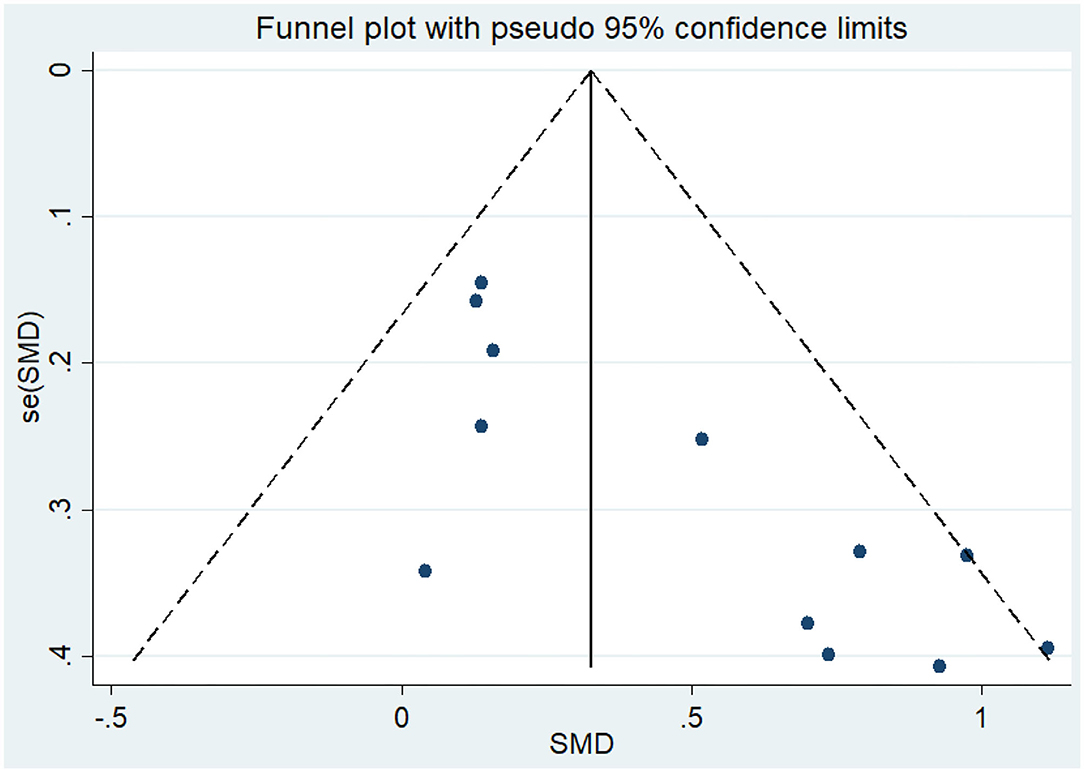
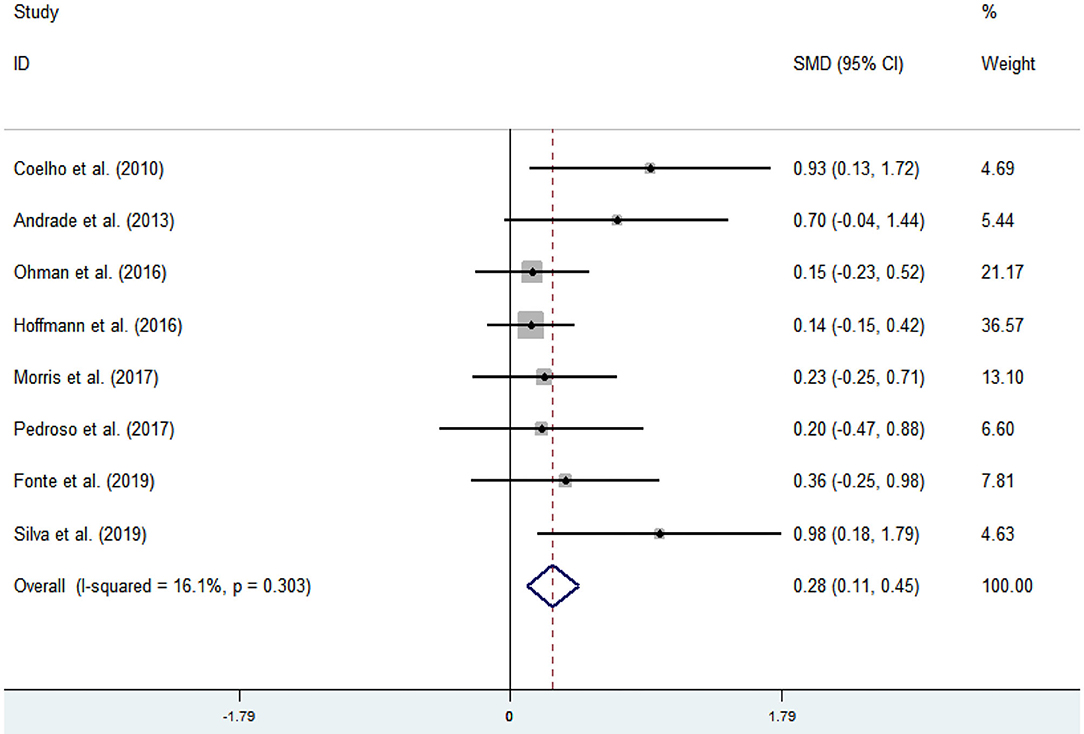
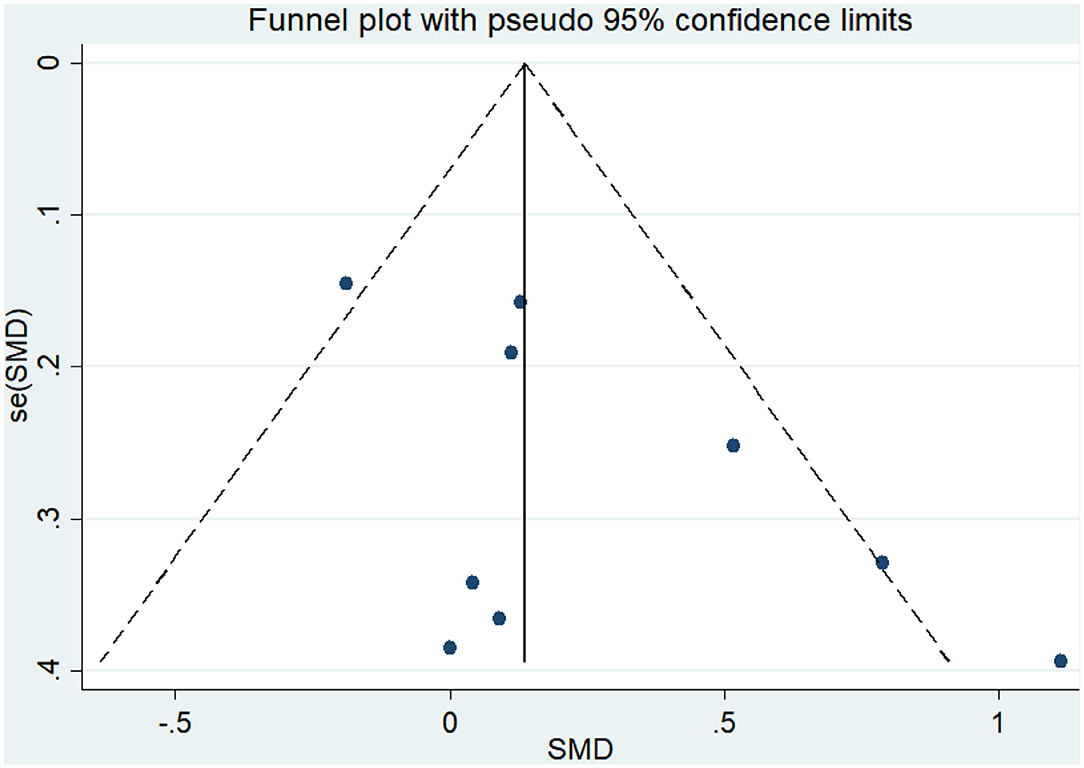
Eight articles (Coelho et al., 2012; Andrade et al., 2013; Hoffmanna et al., 2016; Ohman et al., 2016; Morris et al., 2017; Pedroso et al., 2017; Fonte et al., 2019; Silvaa et al., 2019) evaluated the effects of physical activity on working memory issues in AD patients. An asymmetrical funnel plot was presented. The funnel plot shows that there were no outlier values (Figure 4). The heterogeneity test results of the included research literature were not significant (p = 0.303, I2 = 16.1%); thus, the fixed-effects model was used for meta-analysis (Figure 5). The meta-analysis of eight studies demonstrated that physical activity had significant effects on improving working memory in AD patients (SMD = 0.28, 95% CI 0.11–0.45, p < 0.05).
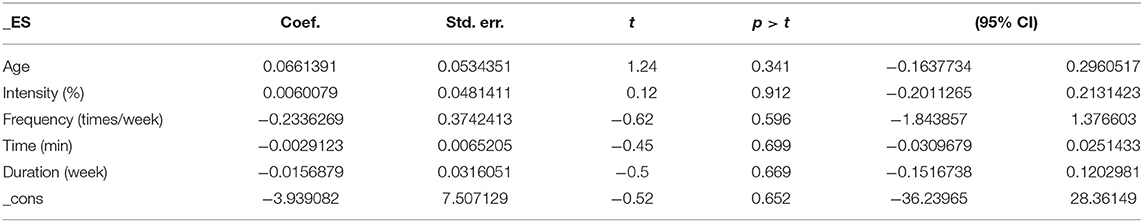
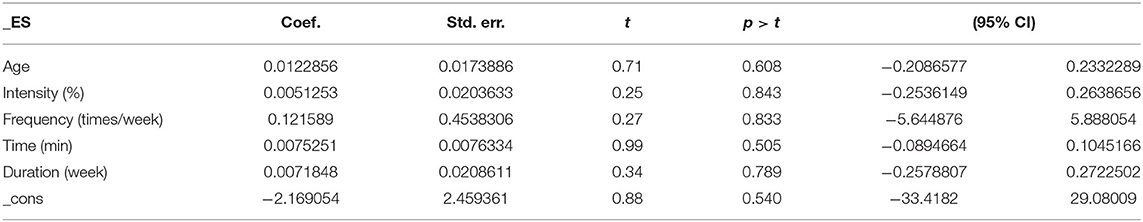
The results of the regression of covariates for cognitive flexibility issues in AD patients are presented in Table 5. For cognitive flexibility, there were no significant effects of age (95% CI −0.1637734 to 0.2960517, p = 0.341), intensity (95% CI −0.2011265 to 0.2131423, p = 0.912), frequency (95% CI −1.843857 to 1.376603, p = 0.596), time (95% CI −0.0309679 to 0.0251433, p = 0.699), or duration (95% CI −0.1516738 to 0.1202981, p = 0.669).
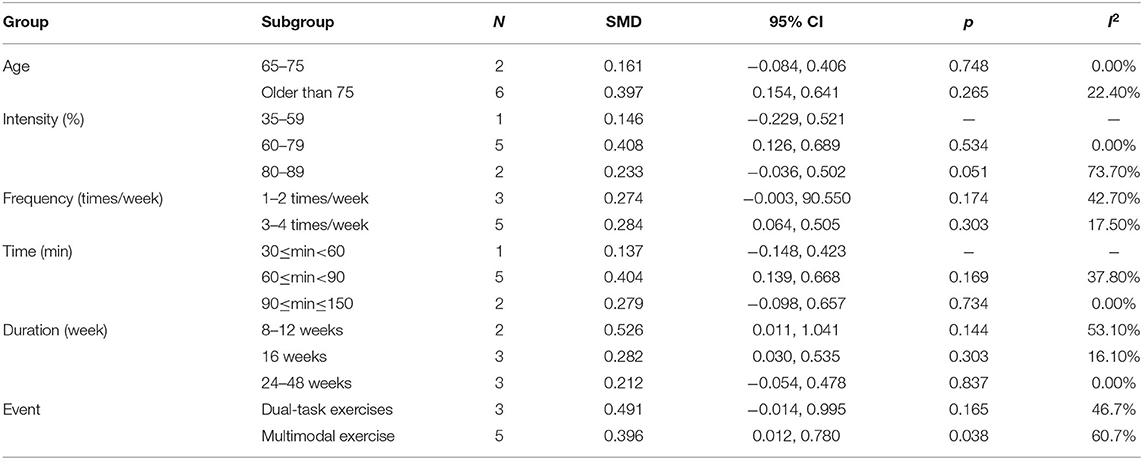
All eligible studies were analyzed in subgroups based on age, intensity, frequency, time, and duration, as shown in Table 6. The results from subgroup analysis are as follows: (1) age: physical activity was beneficial for AD patients aged 65–75 years with working memory issues. (2) Intensity: for working memory issues in AD patients, maintaining a 60–79% MHR during physical activity was more effective. (3) Frequency: the frequency of physical activity has a significant impact on the improvement of the working memory issues in AD patients, and the effect of 3–4 times/week was better than the effect of 1–2 times/week. (4) Time: a total of 60–150 min per exercise session significantly improved working memory in AD patients. (5) Duration: an intervention duration of 16 or 24–48 weeks showed a significant effect on working memory issues in AD patients. (6) Event: for working memory issues in AD patients, dual-task exercises had a significant effect.
Effect of Physical Activity on Cognitive Flexibility Issues in AD Patients
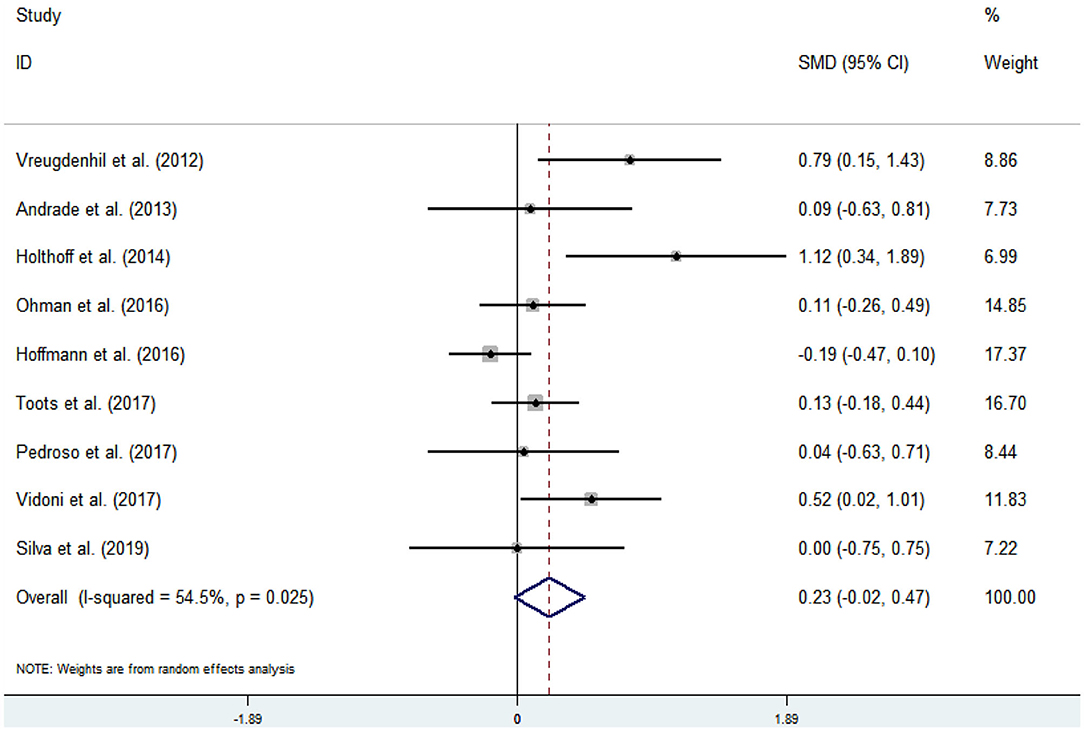
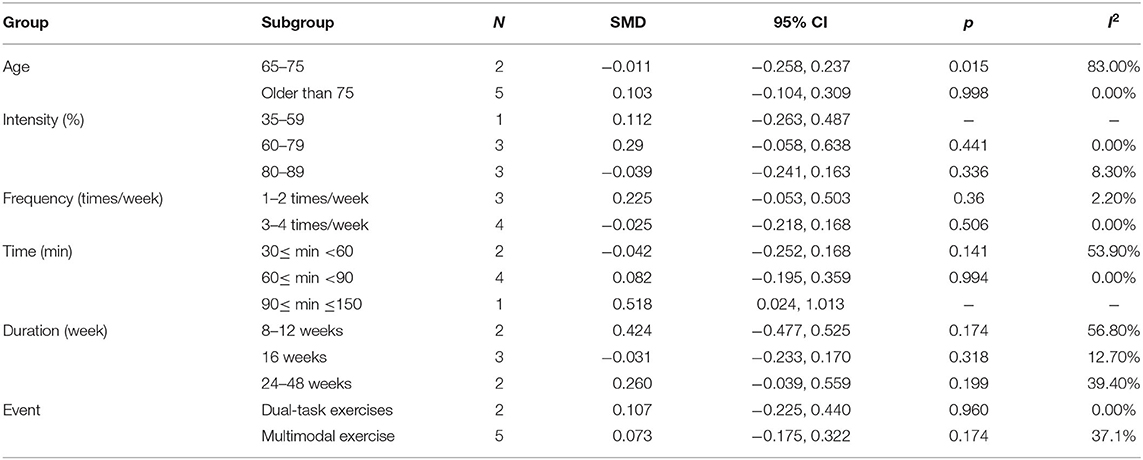
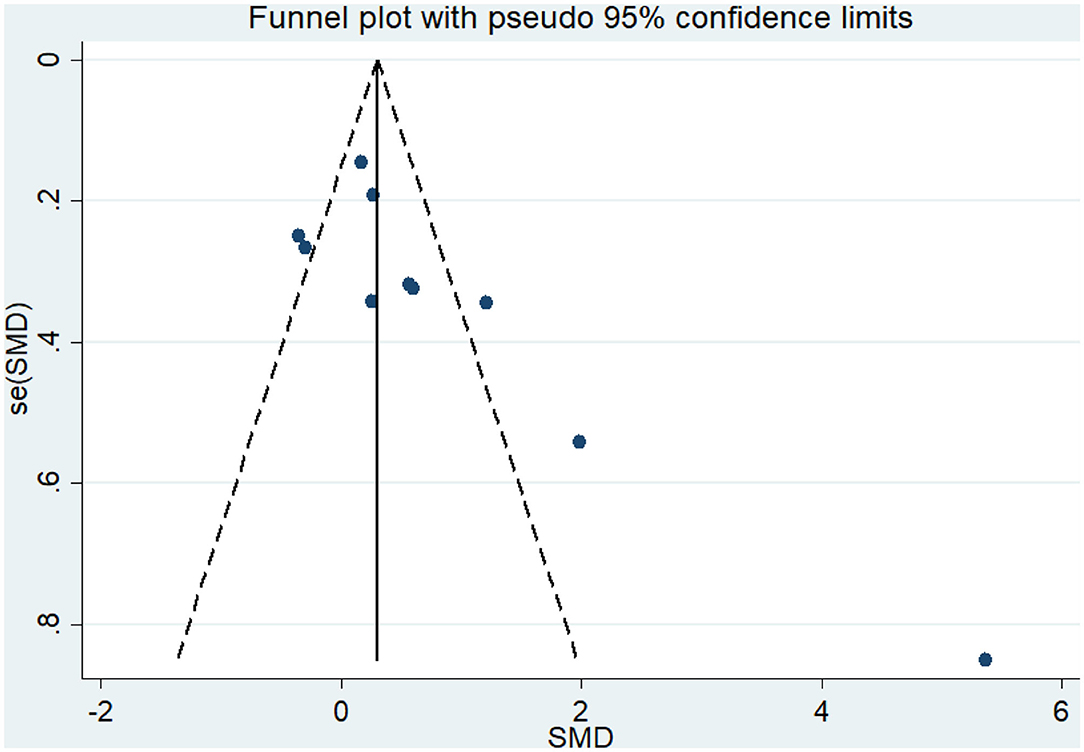
Nine articles (Vreugdenhill et al., 2012; Andrade et al., 2013; Holthoff et al., 2015; Hoffmanna et al., 2016; Ohman et al., 2016; Pedroso et al., 2017; Tootsa et al., 2017; Vidoni et al., 2017; Silvaa et al., 2019) evaluated the effects of physical activity on cognitive flexibility issues in AD patients. An asymmetrical funnel plot was presented. According to the funnel plot, there were two outliers (Figure 6). There was a moderate heterogeneity in the research literature (p = 0.025, I2 = 54.5%), and the meta-analysis was performed using a random-effects models (Figure 7). Figure 7 shows that the meta-analysis of nine studies demonstrated that physical activity had a significant effect on improving cognitive flexibility in AD patients (SMD = 0.23, 95% CI −0.02 to 0.47, p < 0.01).
The analysis of heterogeneity and sensitivity showed that there was considerable bias in the studies by Vreugdenhill et al. (2012) and Holthoff et al. (2015). Therefore, a meta-analysis of the remaining RCTs was performed after exclusion. The results showed that the heterogeneity was reduced (I2 = 7.4%, p = 0.372, SMD = 0.06, 95% CI −0.11, 0.23), and that the differences between groups were significant (p < 0.01).
The results of the regression of covariates for working memory issues in AD patients are presented in Table 7. For working memory, there were no significant effects of age (95% CI −0.2086577 to 0.2332289, p = 0.608), intensity (95% CI −0.2536149 to 0.2638656, p = 0.843), frequency (95% CI −5.644876 to 5.888054, p = 0.833), time (95% CI −0.0894664 to 0.1045166, p = 0.505), or duration (95% CI −0.2578807 to 0.2722502, p = 0.789).
The results from the subgroup analysis are as follows (Table 8): (1) age: physical activity was beneficial for cognitive flexibility issues in AD patients who were older than 75 years. (2) Intensity: for cognitive flexibility issues in AD patients, the effect of maintaining a 60–79 or 80–89% MHR was more effective. (3) Frequency: for cognitive flexibility issues in AD patients, the effect of performing physical activity 1–2 or 3–4 times/week was more effective. (4) Time: a total of 60–90 min per exercise session significantly improved cognitive flexibility in AD patients. (5) Duration: an intervention duration of 16 or 24–48 weeks showed a significant effect on cognitive flexibility issues in AD patients. (6) Event: for cognitive flexibility issues in AD patients, both dual-task exercises and multimodal exercise had significant effects.
Effect of Physical Activity on ADL Issues in AD Patients
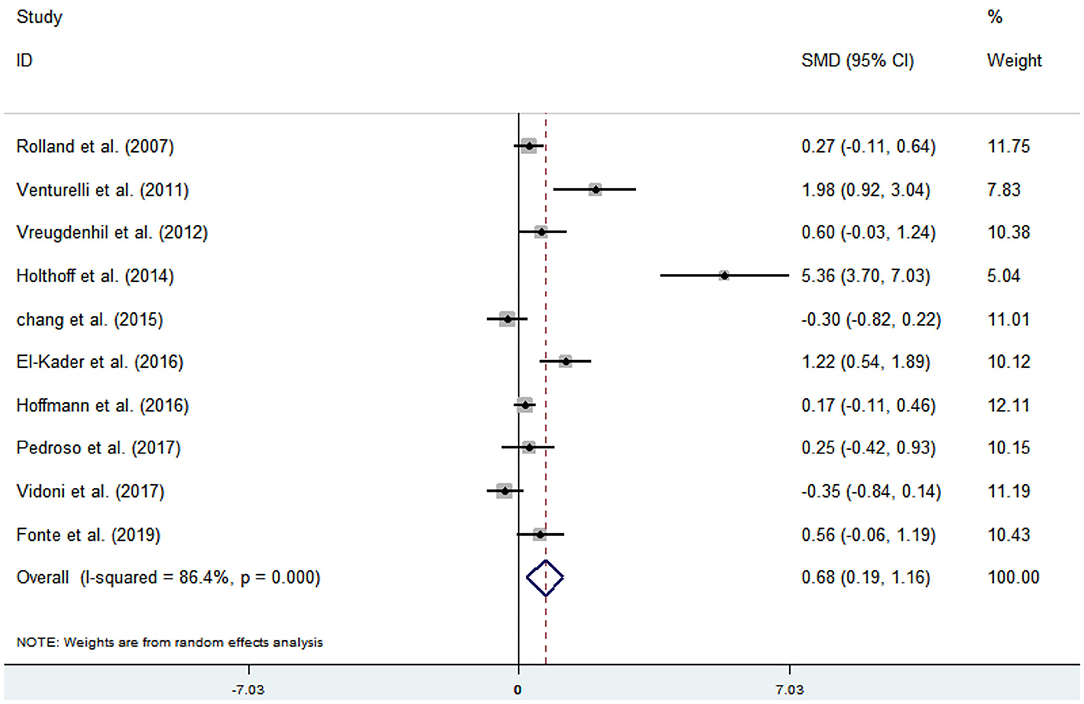
Ten articles (Venturelli et al., 2011; Vreugdenhill et al., 2012; Chang et al., 2015; Holthoff et al., 2015; El-Kader and Al-Jiffr, 2016; Hoffmanna et al., 2016; Pedroso et al., 2017; Rolland et al., 2017; Vidoni et al., 2017; Fonte et al., 2019) evaluated the effects of physical activity on ADL issues in AD patients. An asymmetrical funnel plot was presented. Funnel plot indicated that there were three outliers (Figure 8). Heterogeneity testing of the study literature showed high heterogeneity (p < 0.000, I2 = 86.4%), and a random-effects model was used for the meta-analysis (Figure 9). Figure 9 shows that the meta-analysis of 10 studies demonstrated that physical activity had significant effects on improving ADLs in AD patients (SMD = 0.68, 95% CI 0.19–1.16, p < 0.001).
The analysis of heterogeneity and sensitivity showed that there was considerable bias in the studies by Venturelli et al. (2011), Holthoff et al. (2015), and El-Kader and Al-Jiffr (2016) thus, a meta-analysis of the remaining RCTs was performed after exclusion. The results showed that the heterogeneity was reduced (I2 = 45.1%, p = 0.091, SMD = 0.15, 95% CI −0.10, 0.39), and that the differences between groups were significant (p < 0.001).
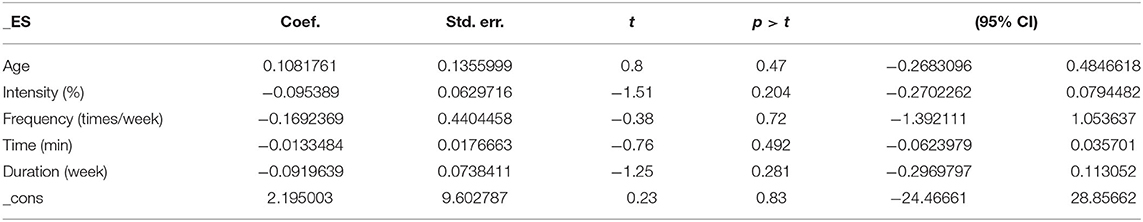
The results of the regression of covariates for ADL issues in AD patients are presented in Table 9. For ADLs, there were no significant effects of age (95% CI −0.2683096 to 0.4846618, p = 0.47), intensity (95% CI −0.2702262 to 0.0794482, p = 0.204), frequency (95% CI −1.392111 to 1.053637, p = 0.72), time (95% CI −0.0623979 to 0.035701, p = 0.492), or duration (95% CI −0.2969797 to 0.113052, p = 0.281).
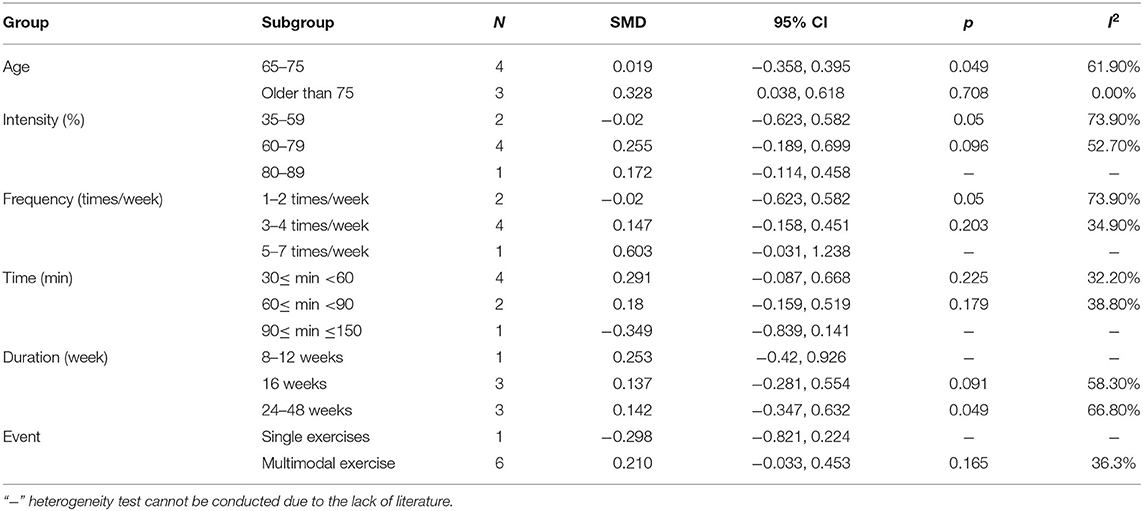
The results from the subgroup analysis are as follows (Table 10): (1) age: physical activity was beneficial for ADL issues in AD patients who were older than 75 years. (2) Intensity: for ADL issues in AD patients, the effect of maintaining a 60–79% MHR was more effective. (3) Frequency: for ADL issues in AD patients, the effect of performing physical activity 3–4 times/week was more effective. (4) Time: a total of 30–60 or 60–90 min per exercise session significantly improved the ADL issues in AD patients. (5) Duration: for ADL issues in AD patients, the intervention duration was not important. (6) Event: for ADL issues in AD patients, multimodal exercise was significant.
Discussion
The purpose of this systematic review and meta-analysis was to compile and analyze the literature pertaining to RCTs on physical activity (e.g., aerobic exercise, aerobic fitness, walking, cycling, strength training, balance training, and flexibility training) in relation to its influence on executive function, working memory, cognitive flexibility, and ADLs among AD patients to determine an optimal exercise prescription. The results suggest that physical activity can improve executive function, working memory, cognitive flexibility, and ADL issues in AD patients.
This systematic review investigated the influence of physical activity on executive function and ADL issues in AD patients. Research suggests that the effect size of executive function was 0.22–0.62 (p < 0.05), that of working memory was 0.11–0.45 (p < 0.05), that of cognitive flexibility was −0.02 to 0.47 (p < 0.01), and that of ADLs was 0.19–1.16 (p < 0.001). Furthermore, significant heterogeneity may have been present in the study by Holthoff et al. (2015). In this study, physical activity was conducted on comfortable chairs to encourage patients to participate and prevent falls and other injuries. This method was different from those used in other studies and may be the cause of the heterogeneity.
The subgroup analysis showed that, for executive function issues, performing moderate-to-high-intensity dual-task exercises or multimodal exercise for more than 60 min per session for 16 weeks had a greater effect on AD patients. Regarding frequency, performing physical activity 1–2 times/week was found to be significant. In addition, for working memory and cognitive flexibility issues, performing 60–90 min of moderate-intensity dual-task exercises 1–4 times/week was more effective. The subgroup analysis indicated that, for ADL issues, maintaining a 60–79% MHR during multimodal exercise 3–4 times/week for 30–90 min each time had a greater effect on AD patients. In addition, in the subgroup analyses of executive function, working memory, cognitive flexibility, and ADL issues, maintaining a 35–59% MHR during physical activity did not show a strong effect compared with maintaining a 60–79 or an 80–89% MHR. Performing physical activity 1–4 times/week for 60–90 min per exercise session significantly improved executive function, working memory, cognitive flexibility, and ADL issues in AD patients. In addition, this meta-analysis provides evidence to support the inclusion of aerobic training and strength, flexibility, balance, and other physical activities to improve executive function and ADL issues in AD patients. For AD patients with executive function, working memory, cognitive flexibility, and ADL issues, both dual-task exercises and multimodal exercises had significant effects. Notably, for working memory and cognitive flexibility issues, dual-task exercises showed a strong influence compared with multimodal exercises. Coincidentally, our findings support the WHO recommendations (World Health Organization Physical Activity Older Adults, 2017). In addition, the characteristics of exercise intervention in the included studies support the frequency, intensity, and time per session recommended by the WHO, but slightly lower than the WHO recommendations based on expert advice. Our meta-analysis showed that the current WHO recommendations can be considered an effective exercise prescription for AD patients; however, future studies are needed to determine which combinations of intervention methods, intensity, duration of each intervention session, frequency, and duration best improve executive function and ADL issues among older adults diagnosed with AD.
Systematic reviews showed that physical activity can improve the attention, cognitive function, executive function, and language of AD patients (Coelho et al., 2009; Farina et al., 2014). These authors agree that there is not enough theoretical support for the ideal intervention program, and that there is no consensus on the intensity, frequency, and duration of exercise. However, long-term physical activity stimulates growth factors, neurotransmitter synthesis, oxygenation, and plasticity to produce nerve and neuroprotective effects on the brain (Deslandes et al., 2009). The beneficial effects of physical activity on brain aging or dementia have not been well-documented (Herholz et al., 2013). However, animal studies have revealed that the activation of adult neurogenesis (Kempermann et al., 2010) or the increase in plasma levels of neuroplasticity-related brain-derived neurotrophic factors (Coelho et al., 2014) is related to physical activity. Furthermore, some mechanisms related to physical activity help to improve cognitive function, such as improvement of the nervous system, including promoting the synthesis of neurotransmitters and improving cerebral blood flow (Eggermont et al., 2006; Lista and Sorrentin, 2009). Some studies have shown that physical activity can increase brain-derived neurotrophic factor and also have a positive impact on the brain neuroplasticity. Kramer and Erickson found that in rodents, physical activity induces increased levels of brain-derived neurotrophic factor in the frontal cortex, hippocampus, and cerebellum and promotes the formation of new capillaries in these areas (Kramer and Erickson, 2007). Some authors believe that physical activity improves cerebral circulation by increasing cerebral blood flow and oxygen supply (Hamer and Chid, 2009; Sofifi et al., 2011), while promoting cardiovascular and cerebrovascular health by lowering blood pressure and blood lipid levels. Physical activity also promotes inflammatory markers and can effectively enhance endothelial function (Kivipelto et al., 2005). Furthermore, physical activity can stimulate the proliferation of neurons in the hippocampus (Erickson et al., 2011). Therefore, the effect of physical activity on clinical performance in AD (e.g., cognitive and executive functions) may rely on improvement in brain functionality.
Previous studies have shown that physical activity can improve the cognitive and executive functions of the elderly with cognitive impairment (Scherder et al., 2005; Lautenschlager et al., 2008; Uffelen et al., 2008; Baker et al., 2010; Lam et al., 2011). The executive function is mainly responsible for the self-regulation of behavior and is the key cognitive resource, including the ability to initiate, plan, sequence, and monitor (Miyake and Friedma, 2012). In AD patients, executive dysfunction is a prominent clinical symptom that directly affects the patient's self-regulation of behavior and ADLs (Boyle et al., 2003; Razani et al., 2007).
The promoting effect of non-pharmacological treatment methods on the improvement of the condition of AD patients has been confirmed (Morley and Silve, 1995; Cohen-Mansfifield and Mintze, 2005). Interventions, such as physical activity, have been shown to improve cognitive function and executive function and are even effective for frail residents of nursing homes (Lazowski et al., 1999). At the same time, exercise intervention may produce effective protective mechanisms to prevent the decline in the ADLs. At present, it has been confirmed that suitable physical activities can inhibit the pathophysiological changes of AD by regulating the expression and hydrolysis of amyloid precursor protein, reducing the production of β amyloid protein, and scavenging free radicals, among other actions (Radak et al., 2010; Foster et al., 2011). In addition, on the one hand, the decline of ADLs among AD patients is due to a decline in the cognitive level; on the other hand, it is closely related to the atrophy of muscles with the development of age and disease (Sakuma and Yamaguch, 2011). Physical activity is an important measure to combat skeletal muscle atrophy. It can increase the quality of skeletal muscle, output power of physical activity per unit time, and skeletal muscle endurance. According to the changes in muscle stimulation during physical activity, the number of activated transverse bridges can be increased, thereby enhancing muscle flexibility (Jin et al., 2020). Furthermore, the contraction ability of respiratory muscles is enhanced after exercise, which is conducive to the extension of patient's trunk and limbs, and provides opportunities for improving limb coordination and strengthening exercise ability (Liu et al., 2018). Therefore, physical activity can improve ADLs in AD patients by increasing the quality of skeletal muscle, enhancing muscle activity, improving limb coordination, and exercise performance, etc.
In this review, one article showed that there was no significant effect of physical activity on executive function among AD patients. Specifically, Morris et al. (2017) showed that there was no significant difference in the improvement of executive function between the intervention group and the control group. Aerobic exercise interventions were used in this study, which are different from other interventions. This may be one of the reasons for the lack of a significant difference. However, aerobic exercise interventions were also used in the study by Vidoni et al. (2017), and the results showed a significantly different effect on ADL issues in AD patients. This result suggests that aerobic exercise may be more effective in improving the quality and activity of skeletal muscle in AD patients. In general, more RCTs are required to demonstrate the effect of aerobic exercise on executive function and ADL issues in AD patients. In addition, other studies have shown that physical activity can improve executive function and ADL issues in AD patients. Therefore, physical activity is feasible and may provide an alternative or adjuvant treatment for patients with mild to moderate or even late AD and their family nurses.
Limitations
There are some limitations and deficiencies in this study. The authenticity and reliability of the results are affected by the course of disease and the frequency, time, intensity, and duration of the intervention in AD patients. There are certain differences in the quality of the eligible articles for our study: (1) only four studies showed their concealed allocation, which may be one of the reasons for the systematic bias of results (PEDro-scale, 2010). (2) Only four articles referred to blinding of assessors. (3) Most of the studies lacked a description of the course of the disease, which could have led to a lack of understanding of the effectiveness of physical activity on executive function and ADL issues in AD patients. (4) Most studies did not mention the severity of disease among patients with AD, which made it impossible to provide patients with more targeted exercise prescriptions. (5) Only two authors, Hoffmanna et al. (2016) and Silvaa et al. (2019), mentioned the effect of physical activity on inhibitory functions in AD patients. Studies on inhibitory executive functions among AD patients are scarce and were not included in the meta-analysis for the time being to avoid errors. Furthermore, the differences in experimental intensity, time, frequency, duration, and outcome measure methods could have led to differences in outcomes and caused difficulty in explanations.
Conclusions
Research has proven that physical activity can effectively improve executive function, working memory, cognitive flexibility, and ADL issues in AD patients and may be an alternative or auxiliary treatment. In the future, it will necessary to provide more accurate neuropsychological evaluations of each executive function, working memory, cognitive flexibility, and ADL dimension and to grade the course of AD to obtain more accurate exercise prescriptions for physical activity interventions. In the absence of adverse reactions, medical profession could combine physical activity with daily medical treatment to optimize the treatment of executive function, working memory, cognitive flexibility, and performance of ADLs in AD patients. In addition, the conclusion of this study still needs to be confirmed by a high-quality large-sample RCT.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary materials, further inquiries can be directed to the corresponding author/s.
Author Contributions
LW and LZ contributed to the idea and structural design plan for review. XJ and HZ applied the search strategy. LZ, LW, and LL applied the selection criteria to screen out qualified documents. LW and XJ completed the deviation risk assessment. LZ wrote the manuscript. LW, LL, and HZ edited the manuscript. All authors analyzed and interpreted the data. All authors have read the complete manuscript and reached a consensus on the manuscript version.
Funding
This research was funded by the National Social Science Foundation (19BTY116).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Allal, G., Annweiler, C., Blumen, H. M., Callisaya, M. L., De-Cock, A. M., Kressig, R. W., et al. (2015). Gait phenotype from mild cognitive impairment to moderate dementia: results from the GOOD initiative. Eur. J. Neurol. 23, 527–541. doi: 10.1111/ene.12882
Andrade, L. P., Gobb, L. T. B., Coelh, F. G. M., and Christofolett, G. (2013). Benefifits of multimodal exercise intervention for postural control and frontal cognitive functions in individuals with alzheimer's disease: a controlled trial. JAGS 61, 1919–1926. doi: 10.1111/jgs.12531
Avilla, R., and Miotto, E. (2002). Funcoes executivas no envelhecimento normal e na doenca de Alzheimer. J. Bras. Psiquiatr. 52, 53–63. Available online at: http://www.ipub.ufrj.br/#jbp.html
Baker, L. D., Fran, L. L., Foster-Schuber, K., Gree, P. S., Wilkinso, C. W., and McTierna, A. (2010). Effects of aerobic exercise on mild cognitive impairment: a controlled trial. Arch. Neurol. 67, 71–79. doi: 10.1001/archneurol.2009.307
Bell-McGinty, S., Podell, K., Franzen, M., Baird, A. D., and Williams, M. J. (2002). Standard measures of executive function in predicting instrumental activities of daily living in older adults. Int. J. Geriatr. Psychiatry 17, 828–834. doi: 10.1002/gps.646
Boyle, P. A., Mallo, P. F., Sallowa, S., Cahn-Weine, D. A., Cohe, R., and Cumming, J. L. (2003). Executive dysfunction and apathy predict functional impairment in alzheimer disease. J. Geriatr. Psychiatry 11, 214–221. doi: 10.1097/00019442-200303000-00012
Cahn-Weiner, D. A., Boyle, P. A., and Malloy, P. F. (2002). Tests of executive function predict instrumental activities of daily living in community-dwelling older individuals. Appl. Neuropsychol. 9, 187–191. doi: 10.1207/S15324826AN0903_8
Chang, C. H., Wan, W., Zh, Y., and Yan, S. Y. (2015). Study on the intervention of aerobic training on alzheimer's disease. Chin. J. Rehabil. Med. 11, 1131–1134. doi: 10.3969/j.issn.1001-1242.2015.11.007
Coelho, F., Vital, T. M., Stei, A. M., Arante, F. J., Rued, A. V., and Camarin, R. (2014). Acute aerobic exercise increases brain derived neurotrophic factor levels in elderly with alzheimer's disease. J. Alz. Dis. 39, 401–408. doi: 10.3233/JAD-131073
Coelho, F. G. M., Andrad, L. P., and Pedros, R. V. (2012). Multimodal exercise intervention improves frontal cognitive functions and gait in alzheimer's disease: a controlled trial. Geriatr. Gerontol. Int. 4, 1–6. doi: 10.1111/j.1447-0594.2012.00887.x
Coelho, F. G. M., Galduróz-Santos, R. F., Gobbi, S., and Stella, F. (2009). Atividade físicasistematizada e desempenho cognitivo com demência de alzheimer: uma revisão sistemática. Revis. Brasileira Psiquiatria. 31, 163–170. doi: 10.1590/S1516-44462009000200014
Cohen-Mansfifield, J., and Mintze, J. E. (2005). Time for change: the role of nonpharmacological interventions in treating behavior problems in nursing home residents with dementia. Alzheimer Dis. Assoc. Disord. 19, 37–40. doi: 10.1097/01.wad.0000155066.39184.61
Davenport, M. H., Hogan, D. B., Eskes, G. A., Longman, R. S., and Poulin, M. J. (2012). Cerebrovascular reserve: the link betweenfifit ness and cognitive function? Exerc. Sport Sci. Rev. 40, 153–158. doi: 10.1097/JES.0b013e3182553430
Deslandes, A., Moraes, H., Ferreira, C., Veiga, H., Silveira, H., Mouta, R., et al. (2009). Exercise and mental health: many reasons to move. Neuropsychobiology 59, 191–198. doi: 10.1159/000223730
Eggermont, L., Swaa, D., Luite, P., and Scherde, E. (2006). Exercise, cognition and alzheimer's disease: more is not necessarily better. Neurosci. Biobehav. Rev. 30, 562–575. doi: 10.1016/j.neubiorev.2005.10.004
El-Kader, S. M., and Al-Jiffr, O. H. (2016). Aerobic exercise improves quality of life, psychological well-being and systemic inflammation in subjects with alzheimer's disease. Afr. Health Sci. 16, 1045–1055. doi: 10.4314/ahs.v16i4.22
Erickson, K. I., Vos, M. W., and Prakas, R. S. (2011). Exercise training increases size of hippocampus and improves memory. Proc. Natl. Acad. Sci. U.S.A. 108, 3017–3022. doi: 10.1073/pnas.1015950108
Erickson, K. I., Weinstein, A. M., and Lopez, O. L. (2012). Physical activity, brain plasticity and alzheimer's disease. Arch. Med. Res. 43, 615–621. doi: 10.1016/j.arcmed.2012.09.008
Farina, F., Rusted, J., and Tabet, N. (2014). The effect of exercise interventions on cognitive outcome in alzheimer's disease: a systematic review. Int. Psychogeriatr. 26, 9–18. doi: 10.1017/S1041610213001385
Fonte, C., Smani, S., Pedrinoll, A., Munari, D., Gandolf, M., and Picell, A. (2019). Comparison between physical and cognitive treatment in patients with MCI and alzheimer's disease. Aging 5, 3138–3155. doi: 10.18632/aging.101970
Foster, P. P., Rosenblatt, K. P., and Kuljiš, R. O. (2011). Exercise-induced cognitive plasticity, implications for mild cognitive impairment and alzheimer's disease. Front. Neurol. 2:28. doi: 10.3389/fneur.2011.00028
Hamer, M., and Chid, Y. (2009). Physical activity and risk of neurodegenerative disease. A systematic review of prospective evidence. Psychol. Med. 39, 3–11. doi: 10.1017/S0033291708003681
Herholz, S. C., Herhol, R. S., and Herhol, K. (2013). Non-pharmacological interventions and neuroplasticity in early stage alzheimer's disease. Expert Rev. Neurother. 13, 1235–1245. doi: 10.1586/14737175.2013.845086
Higgins, J. P. T., Thompso, S. G., Deek, J. J., and Altma, D. G. (2003). Measuring inconsistency in metaanalyses. BMJ Br. Med. J. 327, 557–560. doi: 10.1136/bmj.327.7414.557
Hoffmanna, K., Sobolb, N. A., Frederiksena, K. S., Beyerb, N., Vogela, A., and Vestergaard, K. (2016). Moderate-to-high intensity physical exercise in patients with alzheimer's disease: a randomized controlled trial. J. Alz. Dis. 50, 443–453. doi: 10.3233/JAD-150817
Holthoff, V. A., Marschne, K., Schar, M., Stedin, J., and Meye, S. (2015). Effects of physical activity training in patients with alzheimer's dementia: results of a pilot RCT study. PLoS ONE 4:e0121478. doi: 10.1371/journal.pone.0121478
Jekel, K., Damian, M., and Wattmo, C. (2015). Mild cognitive impairment and defificits in instrumental activities of daily living: a systematic review. Alzheimer Res. Ther. 7:17. doi: 10.1186/s13195-015-0099-0
Jin, X. H., Wan, L., Li, S. J., Zh, L., Loprinz, D., and Fa, X. (2020). The impact of mind-body exercises on motor function, depressive symptoms, and quality of life in parkinso's disease: a systematic review and meta-analysis. Int. J. Environ. Res. Public Health. 17:31. doi: 10.3390/ijerph17010031
Kempermann, G., Fabe, K., Ehninge, D., Bab, H., Leal-Galici, P., and Garth, A. (2010). Why and how physical activity promotes experience-induced brain plasticity. Front. Neurosci. 4:189. doi: 10.3389/fnins.2010.00189
Kivipelto, M., Ngand, T., and Fratiglion, L. (2005). Obesity and vascular risk factors at midlife and the risk of dementia and alzheimers disease. Arch. Neurol. 62, 1556–60. doi: 10.1001/archneur.62.10.1556
Kramer, A. F., and Erickson, K. I. (2007). Capitalizing on cortical plasticity: inflfluence of physical activity on cognition and brain function. Trends Cogn. Sci. 11, 342–348. doi: 10.1016/j.tics.2007.06.009
Lam, L. C., Cha, R. C., Won, B. M., Fun, A. W., Lu, V. W., and Ta, C. C. (2011). Interim follow-up of a randomized controlled trial comparing Chinese style mind body (Tai Chi) and stretching exercises on cognitive function in subjects at risk of progressive cognitive decline. Int. J. Geriatr. Psychiatry 26, 733–740. doi: 10.1002/gps.2602
Lautenschlager, N. T., Co, K. L., Flicke, L., Foste, J. K., Bockxmee, F. M., and Xia, J. (2008). Effect of physical activity on cognitive function in older adults at risk for alzheimer disease: a randomized trial. J. Am. Med. Assoc. 300, 1027–1037. doi: 10.1001/jama.300.9.1027
Lazowski, D. A., Ecclestone, N. A., and Myers, A. M. (1999). A randomized outcome evaluation of group exercise programs in long-term care institutions. J. Gerontol. A Biol. Sci. Med. Sci. 54A, M621–M628. doi: 10.1093/gerona/54.12.M621
Lista, I., and Sorrentin, G. (2009). Biological mechanisms of physical activity in preventing cognitive decline. Cell. Mol. Neurobiol. 30, 493–503. doi: 10.1007/s10571-009-9488-x
Liu, S. J., Ren, Z. B., Wang, L., Wei, G. X., and Zou, L. Y. (2018). Mind-body (Baduanjin) exercise prescription for chronic obstructive pulmonary disease: a systematic review with meta-analysis. Int. J. Environ. Res. Public Health. 15:1830. doi: 10.3390/ijerph15091830
Maffei, L., Picano, E., and Andreassi, M. G. (2017). Train the brain consortium. Randomized trial on the effects of a combined physical/cognitive training in aged MCI subjects: the train the brain study. Sci. Rep. 7:39471. doi: 10.1038/srep39471
Miyake, A., and Friedma, N. P. (2012). The nature and organization of individual differences in executive functions: four general conclusions. Curr. Dir. Psychol. Sci. 21, 8–14. doi: 10.1177/0963721411429458
Morley, J. E., and Silve, A. J. (1995). Nutritional issues in nursing home care. Ann. Intern. Med. 123, 850–859. doi: 10.7326/0003-4819-123-11-199512010-00008
Morris, J. K., Vidon, E. D., Johnson, D. K., Scive, A. V., Mahnke, J. D., Hone, R. A., et al. (2017). Aerobic exercise for alzheimer's disease: a randomized controlled pilot trial. PLoS ONE 2:e0170547. doi: 10.1371/journal.pone.0170547
Norton, S., Matthews, F. E., Barnes, D. E., Yaffe, K., and Brayne, C. (2014). Potential for primary prevention of alzheimer's disease: an analysis of population-based data. Lancet Neurol. 13, 788–794. doi: 10.1016/S1474-4422(14)70136-X
Ohman, H., Savikk, N., Strandber, T. E., Kautiaine, H., and Raivi, M. M. (2016). Effects of exercise on cognition: the finnish alzheimer disease exercise trial: a randomized, controlled trial. JAGS 64, 731–738. doi: 10.1111/jgs.14059
PEDro-scale (2010). Available online at: https://www.pedro.org.au/simplified-chinese/pedro-scale/ (accessed August 4, 2010).
Pedroso, R. V., Ayán, C., Frag, F. G., and Silv, T. M. V. (2017). Effects of functional-task training on older adults with alzheimer's disease. J. Aging Phys. Act. 26, 97–105. doi: 10.1123/japa.2016-0147
Prince, M., Bryce, R., Albanese, E., Wimo, A., Ribeiro, W., and Ferri, C. P. (2013). The global prevalence of dementia: a systematic review and metaanalysis. Alzheimers Dement. 9, 63–75.e2. doi: 10.1016/j.jalz.2012.11.007
Radak, Z., Hart, N., and Sarga, L. (2010). Exercise plays a preventive role against alzheimer's disease. J. Alz. Dis. 20, 777–783. doi: 10.3233/JAD-2010-091531
Razani, J., Casa, R., Won, J. T., Lu, P., Aless, C., and Josephso, K. (2007). Relationship between executive functioning and activities of daily living in patients with relatively mild dementia. Appl. Neuropsychol. 14, 208–214. doi: 10.1080/09084280701509125
Reitz, C., Brayne, C., and Mayeux, R. (2011). Epidemiology of alzheimer disease. Nat. Rev. Neurol. 7, 137–152. doi: 10.1038/nrneurol.2011.2
Rolland, Y., Pillar, F., Klapouszcza, A., and Reynis, E. (2017). Exercise program for nursing home residents with alzheimer's disease: a 1-year randomized, controlled trial. J. Am. Geriatr. Soc. 55, 158–165. doi: 10.1111/j.1532-5415.2007.01035.x
Royall, D. R., Chiodo, L. K., and Polk, M. J. (2000). Correlates of disability among elderly retirees with “subclinical” cognitive impairment. J. Gerontol. A Biol. Sci. Med. Sci. 55, M541–M546. doi: 10.1093/gerona/55.9.M541
Sakuma, K., and Yamaguch, A. (2011). The recent understanding of the neurotrophin's role in skeletal muscle adaptation. J. Biomed. Biotechnol. 2011:201696. doi: 10.1155/2011/201696
Scherder, E. J., Van-Paassche, J., Deije, J. B., Van Der Knokk, S., Orlebek, J. F. K., and Burger, I. (2005). Physical activity and executive functions in the elderly with mild cognitive impairment. Aging Ment. Health 9, 272–280. doi: 10.1080/13607860500089930
Silvaa, F. O., Ferreira, J. V., and Plácido, J. (2019). Three months of multimodal training contributes to mobility and executive function in elderly individuals with mild cognitive impairment, but not in those with alzheimer's disease: a randomized controlled trial. Maturitas 126, 28–33. doi: 10.1016/j.maturitas.2019.04.217
Sofifi, F., Valecch, D., and Bacc, D. (2011). Physical activity and risk of cognitive decline. A meta-analysis of prospective studies. J. Intern. Med. 269, 107–117. doi: 10.1111/j.1365-2796.2010.02281.x
Tan, C. C., Yu, J. T., Wang, H. F., Tan, M. S., Meng, X. F., Wang, C., et al. (2014). Effificacy and safety of donepezil, galantamine, rivastigmine, and memantine for the treatment of alzheimer's disease: a systematic review and meta-analysis. J. Alzheimers Dis. 41, 615–631. doi: 10.3233/JAD-132690
Tootsa, A., Littbran, H., Bostro, G., Hornste, C., and Holmberg, H. (2017). Effects of exercise on cognitive function in older people with dementia: a randomized controlled trial. J. Alz. Dis. 60, 323–332. doi: 10.3233/JAD-170014
Uffelen, J. G., Chinapa, M. J., Mechele, W., and Hopman-Roc, M. (2008). Walking or vitamin B for cognition in older adults with mild cognitive impairment? A randomised controlled trial. Br. J. Sports Med. 42, 344–351. doi: 10.1136/bjsm.2007.044735
Venturelli, M., Scarsin, R., and Schen, F. (2011). Six-month walking program changes cognitive and ADL performance in patients with Alzheimer. J. Alzheimers Dis. 26, 381–388. doi: 10.1177/1533317511418956
Vidoni, E. D., Perale, J., Alshehr, M., Gile, A. M., and Siengsuko, C. F. (2017). Aerobic exercise sustains performance of instrumental activities of daily living in early-stage alzheimer disease. J. Geriatr. Phys. Ther. 42, 1–6. doi: 10.1519/JPT.0000000000000172
Vreugdenhill, A., Cannel, J., Davie, A., and Raza, G. (2012). A community-based exercise programme to improve functional ability in people with alzheimer's disease: a randomized controlled trial. Scand. J. Caring Sci. 26, 12–19. doi: 10.1111/j.1471-6712.2011.00895.x
Wilbur, J., Marquez, D. X., Fogg, L., Wilson, R. S., Staffifileno, B. A., Hoyem, R. L., et al. (2012). The relationship between physical activity and cognition in older latinos. J. Gerontol. B Psychol. Sci. Soc. Sci. 67, 525–534. doi: 10.1093/geronb/gbr137
World Health Organization Physical Activity Older Adults (2017). Available online at: http://www.who.int/dietphysicalactivity/factsheet_olderadults/en (accessed January 18, 2017).
Yaari, R., and Bloom, J. C. (2007). Alzheimer's disease. Semin. Neurol. 27, 32–41. doi: 10.1055/s-2006-956753
Keywords: exercise prescription, ADL, executive function, AD, physical activity
Citation: Zhu L, Li L, Wang L, Jin X and Zhang H (2020) Physical Activity for Executive Function and Activities of Daily Living in AD Patients: A Systematic Review and Meta-Analysis. Front. Psychol. 11:560461. doi: 10.3389/fpsyg.2020.560461
Received: 09 May 2020; Accepted: 28 October 2020;
Published: 03 December 2020.
Edited by:
Liye Zou, Shenzhen University, ChinaReviewed by:
Francesca Federico, Sapienza University of Rome, ItalyJeffer Eidi Sasaki, Universidade Federal Do Triângulo Mineiro, Brazil
Copyright © 2020 Zhu, Li, Wang, Jin and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Long Li, lilong@suda.edu.cn; Lin Wang, wanglin123@126.com
 Lin Zhu
Lin Zhu Long Li1*
Long Li1* Xiaohu Jin
Xiaohu Jin