Abstract
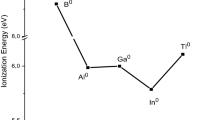
Demarcation of elements for two subsets appears to be the most fundamental approach to their classification. If one draws a vertical straight line through the middle of each block of elements in the Periodic table, all the elements are divided into two subsets: “early” and “later”. For example, in the d-block, the early ones are Sc–Mn, and the late ones, respectively, are Fe–Zn. Later elements partially repeat the properties of the early ones, and this is defined as the internal periodicity. Another criterion for dividing the elements into two subsets is the evenness and oddness of the sum of n + l, where n is the principal quantum number, and l is the orbital quantum number for the outer electron subshells. Properties of the odd elements (for example, B–Ne, Ga–Kr, Tl–Rn in the p-block) are closer to each other than to properties of even elements (Al–Ar, In–Xe), and vice versa. This regularity is manifested as the secondary periodicity. The history of concepts, which considered the existence of subsets as well as of inner and secondary periodicities, is discussed. The features of the electronic structure, which underlie the existence of subsets, are considered. The existence of subsets was depicted earlier by dividing the periodic table into two tables or by applying a mirror-symmetric table. Small changes are proposed in the conventional Periodic table, allowing to reflect the existence of the considered subsets.











Similar content being viewed by others
Notes
Here and below the modern group numbers in the medium-long Periodic Table 1 are given in brackets.
But "anomalous valences" is clearly a chemical property, however is determined by the degree of filling of d- and f-subshells.
This, of course, does not mean that the chemists deny the very phenomenon of internal periodicity.
It should be noted that many symmetric tables have been proposed, which do not have these drawbacks, for example, see the Bayley–Thomsen–Bohr table (Imyanitov (2011c, p. 2190). However, the symmetry line (also implicitly) passes through the middle of the periods (but not through the middle of the rows in blocks) does not divide the elements into early and late in this tables.
Based on the heats of formation of the corresponding compounds.
In modern variants (Table 1), there are no subgroups in periodic tables, subgroups correspond to groups.
According to modern terminology, "secondary periodicity is observed in all groups".
It may be useful to consider Tables 5 as an aggregate of 4-th subsets: early with odd n + l, early with even n + l, late with odd n + 1, late with even n + l.
References
Alternative Periodic Table.: The Chemogenesis web book, The INTERNET Database of Periodic Tables. Curator: M.R. Leach (2017). http://www.meta-synthesis.com/webbook/35_pt/pt_database.php?PT_id=739. Accessed 23 Feb 2017
Balarev, D., Andreev, S.T.: Broader regularity in the periodic system. Annuaire Univ. Sofia. II Fac. Sci. 46 (Livre 2), 159–175 (1950). (in Bulgarian)
Basolo, F., Pearson, R.G.: Mechanisms of Inorganic Reactions: A Study of Metal Complexes in Solution, 2nd edn. Wiley, New York (1967)
Bent, H.: New Ideas in Chemistry from Fresh Energy for the Periodic Law. AuthorHouse, Bloomington (2006)
Berkengejm, A.M.: Theoretical Foundations of Chemistry, p. 146. GIZ, Moskow-Leningrad (1926). (in Russian)
Biltz, W., Klemm, W.: Die Unterteilung der Reichen der Übergangselemente. Ztsch. Elektrochem. 39, 597–598 (1933)
Biron, E.V.: Phenomena of secondary periodicity. Zh. Russ. Fiz.-Khim. Obshch. Ch. Khim. 47, 964–988 (1915). (in Russian)
Bohr, N.: On the constitution of atoms and molecules. Philos. Mag. 1798–1977(26), 1–24 (1913)
Brandt, S., Dahmen, H.D.: The Picture Book of Quantum, 4th edn, p. 251. Springer, New York (2012)
Brauner, B.: Über die Stellung der Elemente der seltenen Erden im periodischen System. Ztsch. Elektrochem. 14, 525–527 (1908)
Burdett, N.A., Hayhurst, A.N.: Determination of the rate coefficients of A + X = A+ + X- and AX + M = A+ + X- + M where A is a metal atom, X a halogen atom and M a flame species. Philos. Trans. Royal Soc. (Lond.) Ser. A. 290, 299–325 (1979)
Cartledge, G.H.: The correlation of thermochemical data by the ionic potential. J. Phys. Colloid Chem. 55, 248–256 (1951)
Cerasoli, E.: The periodic system and Pauli’s exclusion principle. Chimica nell’Industria, nell’Agricoltura, nella Biologia e nelle Realizzazioni Corporative. 17, 37–43 (1941)
Chistyakov, V.M.: “Secondary Periodicity of Biron” in secondary d-subgroups of the short periodic table. Zh. Obshch. Khim. 38, 209–210 (1968). (in Russian)
Didyk, Y. K.: Derivation of the periodic law on the basis of quantum mechanics. The existence of mirror-symmetric sets of elements. In: Sb. Nauchn. Trudov Noril’sk. Vech. Industr. In-ta. Krasnoyarsk. 15, 37–62 (1973). (in Russian)
Didyk, Y.K., Makarenya A.A., Sukhomlinov B.D.: Experimental substantiation of the division of a set of elements into two symmetrical subsets. In: Sadovskij G.I. (ed.) Dobycha i pererabotka rud tsvetnykh metallov, pp. 117–122. Izd-vo Noril’sk. Vech. Industr. In-t., Noril’sk, (1978). (in Russian)
Didyk, Y.K.: Periodic systems of elements, conservation laws and corresponding similarity groups. In: Tyukhtin V.S., Urmantsev Yu.A. (eds.) Sistema. Simmetriya. Garmoniya, pp. 244–260. Mysl’, Moscow (1988). (in Russian)
Didyk, Yu. K., Astaf’eva E.M.: Mirror symmetry in the structure of an atom and periodicity of elements. Khimizdat, Sankt-Peterburg (Russia). (2008). (in Russian)
Frackiewicz, K., Czerwinski, M., Siekierski, S.: Secondary periodicity in the tetrahalogeno complexes of the group 13 elements. Eur. J. Inorg. Chem. 19, 3850–3856 (2005)
Ghanty, T.K., Ghosh, S.K.: Spin-polarized generalization of the concepts of electronegativity and hardness and the description of chemical binding. J. Am. Chem. Soc. 116, 3943–3948 (1994)
Ghosh, S.K.: Electronegativity, hardness, and a semiempirical density functional theory of chemical binding. Int. J. Quant. Chem. 49, 239–251 (1994)
Goldschmidt, V. M., Barth, T., Lunde, G.: Geochemical distribution law of the elements. V. Isomorphy and polymorphy of the sesquioxides. The contraction of the “lanthanums” and its consequences. Skrifter Norske Videnskaps. Akad. Oslo., 1 Mat.-Nat. Kl., 7, 59 pp. (1925). Chem. Abstr. 19, 3391(1925)
Gorbunov, A. I., Filippov, G. G.: Fine Structure of D. I. Mendeleev Periodic Table: secondary periodicity, early and late elements. Khim-ya Tekhnol. 11, 43–45 (2001). (in Russian)
Gurin, V.E.: Element property diagrams of a new form and the phenomenon of secondary periodicity. Zh. Obshch. Khim. 64, 367–370 (1994). (in Russian)
Habashi, F.: Metals: typical and less typical, transition and inner transition. Found. Chem. 12, 31–39 (2010)
Han, F.: A Modern Course in University Physics, p. 588. World Scientific Publishing Co., Singapore (2017)
Hart, D.: Periodicity of chemical thermodynamic functions. J. Phys. Colloid Chem. 56, 202–214 (1952)
Hildebrand, J.H.: The alternations in stability of compounds of the elements of group V. J. Chem. Educ. 18, 291–292 (1941)
Imyanitov, N.S.: Dialectic functions for description and prediction of proton affinity and basicity in gas phase. Russ. J. Org. Chem. 37, 1196–1204 (2011a)
Imyanitov, N.S.: Dialectic functions for description and prediction of proton affinity and basicity in gas phase. Russ. J. Org. Chem. 37, 1196–1204 (2011b). (in Russian)
Imyanitov, N.S.: The periodic law. Formulations, equations, graphic representations. Russ. J. Inorg. Chem. 56, 2183–2200 (2011c)
Imyanitov, N.S.: Adequacy of the new formulation of the Periodic Law when fundamental variations occur in blocks and periods. Found. Chem. 16, 235–247 (2014)
Imyanitov, N.S.: Dialectics and synergetics in chemistry. Periodic Table and oscillating Reactions. Found. Chem. 18, 21–56 (2016a)
Imyanitov, N.S.: Spiral as the fundamental graphic representation of the Periodic Law. Blocks of elements as the autonomic parts of the Periodic System. Found. Chem. 18, 153–173 (2016b)
Janes, R., Moore, E.A.: Metal-Ligand Bonding. Roy. Soc. Chem. Bath Press, Glasgow (2004)
Jørgensen, C.K.: Energy Levels of Complexes and Gaseous Ions, (Ph.D Thesis, University of Copenhagen), Gjellerups Forlag, Copenhagen (1957)
Jorgensen, K.: Oxidation numbers and oxidation states, p. 49. Springer, London (1969)
Kablukov, I.A.: Thermochemistry. ONTI, Moskow-Leningrad (1934). (in Russian)
Kaupp, M.: The role of radial nodes of atomic orbitals for chemical bonding and the periodic table. J. Comput. Chem. 28, 320–325 (2007)
Kerr, J.A.: Strenghts of Chemical Bonds. In: Linde D.R. (ed-in-chief) CRC Handbook of Chemistry and Physics, 85th ed., pp. 9–52–9–64. CRC Press, Boca Raton etc (2004–2005)
Klemm, W.: Eine Systematik der seltenen Erden, begründet auf periodischen Eigenschaftsänderungen ihrer Ionen. Ztschr. anorg. allgem. Chem. 184, 345–351 (1929)
Klemm, W., Bommer, A.: Zur Kenntnis der Metalle der seltenen Erden. Ztschr. anorg. allgem. Chem. 231, 138–171 (1937)
Klemm, W.: Zur Systematik der seltenen Erden. Angew. Chem. 51(575–576), 577–581 (1938)
Klemm, W., Westlinning, H.: Untersuchungen über die Verbindungen der Magnesiums mit den Elementen der IVb-Gruppe. Ztschr. anorg. allgem. Chem. 245, 365–380 (1941)
Klemm, W.: Die Bedeutung “halbbesetzter” Elektronenkonfigurationen für die Chemie. Chemiker Ztg. 66, 365–368 (1942)
Korableva, T.P., Korol’kov, D.V.: Theory of the Periodic System. Izd-vo SPbU, St. Petersburg (2005). (in Russian)
Korol’kov, D.V., Skorobogatov, G.A.: Theoretical Chemistry. 2nd ed. p. 84. Izd-vo SPbU, St. Petersburg (2005). (in Russian)
Kramida, A., Ralchenko, Yu., Reader, J., NIST ASD Team.: NIST Atomic Spectra Database (ver. 5.2), Ground States and Ionization Energies (Online). National Institute of Standards and Technology, Gaithersburg, MD. http://physics.nist.gov/PhysRefData/ASD/ionEnergy.html (2014). Accessed 22 March 2017
Lakatos, B.: Transition metal contraction and double contraction. Naturwissensch 41, 355–356 (1954)
Lakatos, B.: Periodicity of the chemical thermodynamic properties of compounds. Acta Chim. Acad. Scient. Hung. 8, 207–231 (1955)
Li, Jiping, He, H.: Explanation for secondary periodicity using quantum chemical relativistic effects. Huaxue Yanjiu Yu Yingyong 8, 581–584 (1996). (in Chinese)
Magomedov, M.N.: The correlation of the parameters of interatomic interaction in crystals with the position of atom in the periodic table. High Temp. 46, 484–494 (2008)
Mazurs’ 1967 Formulation. The Chemogenesis web book, The INTERNET Database of Periodic Tables. Curator: M.R. Leach (1967). http://www.meta-synthesis.com/webbook/35_pt/pt_database.php?PT_id=298. Accessed 23 Feb 2017
Mazurs, E.G.: Graphic Representations of the Periodic System During One Hundred Years, 2nd edn, p. 127. University of Alabama Press, Ala (1974)
Mel’nikov, V.P., Dmitriev, I.S.: Additional Types of Periodicity in the D. I. Mendeleev’s Periodic System. Nauka, Moscow (1988). (in Russian)
Meyer, R.J.: Die stellung der elemente der seltenen erden im periodischen system. Naturwissensch 2, 781–787 (1914)
Morozova, M.P., Li Myao-syu, Golomolzina M.V.: The enthalpy of formation of strontium compounds with elements of the main subgroup of the IV group. Vestn. Leningr. Gos. Un-ta 10–2, 83–86 (1959). (in Russian)
Mosander, C.: On the new metals, lanthanium and didymium, which are associated with cerium, and on erbium and terbium, new metals associated with yttria. Philos. Mag. [3] 23, 241–254 (1843)
Neubert, D.: Double Shell Structure of the Periodic System of the Elements. Z. Naturforsch. 25a, 210–217 (1970)
Noddak W., Brukl, A.: Zur Klemmschen Systematik der seltenen Erden. Angew. Chem. 51, 576–577, 581(1938)
Odabasi, H.: Some evidence about the dynamical group SO(4, 2) symmetries of the periodic table of elements. Int. J. Quant. Chem. Symp. 7, 23–33 (1973)
Ostrovsky, V.N.: Dynamic symmetry of atomic potential. J. Phys. B. 14, 4425–4439 (1981)
Panchenko, YuN, Abramenkov, A.V., De, George R., Maré, G.R.: Vibrational spectra and ab initio analysis of tert-butyl, trimethylsilyl, trimethylgermyl, trimethylstannyl and trimethylplumbyl derivatives of 3,3-dimethylcyclopropene. XI. Secondary periodicity. Spectrochim. Acta 73A, 782–786 (2009)
Drits M.E. (Ed): Properties of elements, Handbook, 3rd edn., vol 1, pp. 21, 23. Ruda i Metally, Moscow (2003)
Pyykkö, P., Desclaux, J.P.: Relativity and the periodic system of elements. Acc. Chem. Res. 12, 276–281 (1979)
Pyykkö, P.: On the interpretation of ‘secondary periodicity’ in the periodic system. J. Chem. Res. Synopses 11, 380–381 (1979)
Pyykkö, P.: Relativistic effects in structural chemistry. Chem. Rev. 88, 563–594 (1988)
Pyykkö, P.: A note on nodal structures, partial screening, and periodic trends among alkali metals and alkaline earths. Int. J. Quant. Chem. 85, 18–21 (2001)
Rabinowitsch, E., Thilo, E: Periodisches System. Geschichte und Theorie. S, p. 261. F. Enke, Stuttgart (1930)
Roth, W.A., Becker, G.: Ordnungszahl und bildungswärme. Ztschr. Phys. Chem. A. 159, 1–26 (1932)
Rutherford, E.: Scattering of alpha and beta particles of matter and the structure of the atom. Phil. Mag. 1798–1977(21), 669–689 (1911)
Sanderson, R.T.: An explanation of chemical variations within periodic major groups. J. Am. Chem. Soc. 74, 4792–4794 (1952a)
Sanderson, R.T.: Stability of nonpolar covalent bonds. J. Chem. Phys. 20, 535 (1952b)
Scerri, E.R.: Presenting the left-step periodic table. Edu. Chem. 42, 135–136 (2005a)
Scerri, E.R.: Editorial 21. Found. Chem. 7, 199–202 (2005b)
Scerri, E.R.: The periodic table: its story and its significance. Oxford University Press, New York (2007)
Scerri, E.R.: A very short introduction to the periodic table. Oxford University Press, Oxford (2011a)
Scerri, E.R.: A review of research on the history and philosophy of the periodic table. \ Una revisio´n de investigaciones sobre la historia y la filosofı´a de la tabla perio´dica. J. Sci. Educ. 12, 4–7 (2011b)
Scerri, E.: Mendeleev’s periodic table is finally completed and what to do about group 3? Chem. Int. 28–31, July–August (2012)
Scerri, E.R., Parsons, W.: What elements belong in group 3 of the periodic table? In: Scerri E., Restrepo G. (eds.) Mendeleev to oganesson. A multidisciplinary perspective on the periodic table, pp. 140–151. Oxford Univ Press, New York, NY (2018)
Siekierski, S.: Ionic Radii: effect of shell radius, cation charge and lone electron pair. Commun. Inorg. Chem. 19, 121–131 (1997)
Shchukarev, S.A., Vasil’kova, I.V.: The phenomenon of secondary periodicity on the example of magnesium compounds with elements of the main subgroup of the IV group in the D.I. Mendeleev system. Vestn. Leningr. Gos. Un-ta. 2–1, 115–120 (1953). (in Russian)
Shchukarev, S.A.: The periodic properties of electronic orbits of free atoms, and the relation of such periodicity with the properties of elements, chemical compounds, and solutions of electrolytes. Vestn. Leningr. Gos. Un-ta 11–4, 127–151 (1954a). (in Russian)
Shchukarev, S.A.: D. I. Mendeleev’s periodic law as a basic principle of modern chemistry. Zh. Obshch. Khim. 24, 595–603 (1954b)
Shchukarev, S.A.: D. I. Mendeleev’s periodic law as a basic principle of modern chemistry. Zh. Obshch. Khim. 24, 581–592 (1954c). (in Russian)
Shchukarev, S.A., Morozova, M.P., Prokof’eva, E.A.: Higher barium phosphides. Zh. Obshch. Khim. 24, 1261–1262 (1954a)
Shchukarev, S.A., Morozova, M.P., Prokof’eva, E.A.: Higher barium phosphides. Zh. Obshch. Khim. 24, 1277–1278 (1954b). (in Russian)
Shchukarev, S.A., Grossman, G., Morozova, M.P.: The enthalpy of formation of zinc phosphide, Zn3P2. Zh. Obshch. Khim. 25, 607–608 (1955a)
Shchukarev, S.A., Grossman, G., Morozova, M.P.: The enthalpy of formation of zinc phosphide, Zn3P2. Zh. Obshch. Khim. 25, 633–634 (1955b). (in Russian)
Shchukarev, S.A.: Modern significance of D. I. Mendeleev’s periodic law and prospects for development. In: Semenov, N. N. (ed) Sto Let Period. Zakona Khim. Elem., Dokl. Plenarnykh Zased., Yubileinyi Mendeleev. S’ezd, 10th. pp. 40–53. Nauka, Moscow (1971). (in Russian)
Shchukarev, S.A.: New views of D.I. Mendeleev’s system. I. Periodicity of the stratigraphy of atomic electronic shells in the system, and the concept of kainosymmetry. Zh. Obshch. Khim. 47, 246–259 (1977)
Shishokin, V.P.: Secondary periodicity in the periodic chart of D. I Mendeleev. Zh. Obshch. Khim. 23, 929–933 (1953a)
Shishokin, V.P.: Secondary periodicity in the periodic chart of D. I Mendeleev. Zh. Obshch. Khim. 23, 889–893 (1953b). (in Russian)
Thomsen, J.: Systematishe Durchtfuhrung thermochemischer Untersuchunden. S. 152. 160, 171. F. Enke, Stuttgart (1906)
Thyssen, P., Binnemans, K.: Accommodation of the rare earths in the periodic table: a historical analysis. In: Gschneidner, K. A, Jr. (ed) Handbook on the Physics and Chemistry of Rare Earths 41, 1–93 (2011)
Thyssen, P., Ceulemans, A.: Shattered symmetry: group theory from the eightfold way to the periodic table, pp. 380, 412; tabl. 13.7. Oxford University Press, Oxford (2016)
Trifonov, D.N.: Afterword of the editor. In: Mel’nikov, V.P., Dmitriev, I.S.: Additional types of periodicity in the D. I. Mendeleev’s periodic system. Nauka, Moscow (1988). (in Russian)
Urmantsev, YuA: Poly- and isomorphism in living and inanimate nature. Voprosy Filosofii 12, 77–88 (1968). (in Russian)
Urmantsev, Y.A.: Symmetry of system and system of symmetry. Comp. & Maths with Appls. 12B, Iss. 1/2, 379–405 (1986)
Urmantsev, Y.A.: General theory of systems: state, applications and development prospects. In: Tyukhtin V.S., Urmantsev Yu.A. (eds.) Sistema. Simmetriya. Garmoniya. pp. 38–130. Mysl’, Moscow (1988). (in Russian)
Vyatkin, V.B.: Orbital system of distribution of electrons in atom and structure of periodic system of elements. Nauchnyj Zh. Kubansk. Gos. Agrarn. Un-ta. 89 (05), 1–34 (2013). (in Russian)
Wang, S.-G., Schwarz, W.H.E.: Icon of chemistry: the periodic system of chemical elements in the new century. Angew. Chem. Int. Ed. 48, 3404–3415 (2009)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Imyanitov, N.S. Does the period table appear doubled? Two variants of division of elements into two subsets. Internal and secondary periodicity. Found Chem 21, 255–284 (2019). https://doi.org/10.1007/s10698-018-9321-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10698-018-9321-z