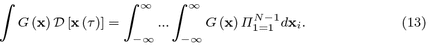Abstract
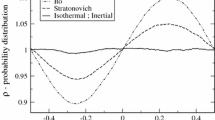
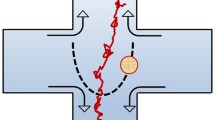
Analytical models describing the motion of colloidal particles in given force fields are presented. In addition to local approaches, leading to well known master equations such as the Langevin and the Fokker–Planck equations, a global description based on path integration is reviewed. A new result is presented, showing that under very broad conditions, during its evolution a dissipative system tends to minimize its energy dissipation in such a way to keep constant the Hamiltonian time rate, equal to the difference between the flux-based and the force-based Rayleigh dissipation functions. In fact, the Fokker–Planck equation can be interpreted as the Hamilton–Jacobi equation resulting from such minumum principle. At steady state, the Hamiltonian time rate is maximized, leading to a minimum resistance principle. In the unsteady case, we consider the relaxation to equilibrium of harmonic oscillators and the motion of a Brownian particle in shear flow, obtaining results that coincide with the solution of the Fokker–Planck and the Langevin equations.
Similar content being viewed by others
Notes
Clearly, this is true only when the system is close to its equilibrium configuration, since, in general, phenomenological coefficients are far from being constant; for example, in the case of heat conduction, thermal conductivity, relating heat current and temperature gradient, should vary with temperature as \(T^{-2}\), which is very unlikely, and so the principle of minimum entropy production should be taken with great caution.
Denoting \( \epsilon = t / N \), \( t_i = i \epsilon \) and \( \mathbf x _i = \mathbf x (t_i) \), the path integral is defined as the following functional integral:
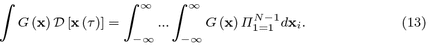
A rigorous and complete treatment on functional integration applied on diffusion processes can be found in Graham [26].
This can also be proved explicitly, multiplying Eq. (23) by \(\dot{\mathbf{y}}\) and considering that \( \frac{d}{dt} = \frac{\partial }{\partial t} + \dot{\mathbf{y}} \cdot \mathbf{\nabla }\), obtaining \( d\mathcal H /dt = 0 \), i.e. \(\mathcal H \) is constant, along the minimum path.
References
Kirchhoff, G.D.: Ueber die Anwendbarkeit der Formeln for die Intensitfiten der galvanischen Strome in einem Systeme linearer leiter auf Systemen, die zum Theil aus nicht linearem Leitern bestehen. Ann. Phys. 75, 189–205 (1848)
Prigogine, I.: Introduction to Thermodynamics of Irreversible Processes, 2nd edn. Interscience, New York (1961)
De Groot, S.R., Mazur, P.: Non-Equilibrium Thermodynamics. North Holland, Amsterdam (1962)
Jaynes, E.T.: The minimum entropy production principle. Ann. Rev. Phys. Chem. 31, 579–601 (1980)
Müller, I., Weiss, W.: Thermodynamics of irreversible processes - past and present. Eur. Phys. J. H 37, 139–236 (2012)
Ziegler, H.: An Introduction to Thermomechanics. North-Holland, Amsterdam (1983)
Hill, R.: The Mathematical Theory of Plasticity. Oxford University Press, Oxford (1950)
Onsager, L.: Reciprocal relations in irreversible processes II. Phys. Rev. 38, 2265–2279 (1931)
Onsager, L., Machlup, S.: Fluctuations and irreversible processes. Phys. Rev. 91, 1505–1512 (1953)
Gyarmati, I.: Non-Equilibrium Thermodynamics. Springer, Heidelberg (1970)
Zupanović, P., Yuretić, D., Botrić, S.: Kirchhoff’s loop law and the maximum entropy production principle. Phys. Rev. E 70, 056108 (2004)
Martyushev, L.M., Seleznev, V.D.: Maximum entropy production principle in physics, chemistry and biology. Phys. Rep. 426, 1–45 (2006)
Dewar, R.C.: Maximum entropy production and the fluctuation theorem. J. Phys. A 38, L371–L381 (2005)
Favretti, M.: The maximum entropy rate description of a thermodynamic system in a stationary non-equilibrium state. Entropy 11, 675–687 (2009)
Polettini, M.: Fact-checking Ziegler’s maximum entropy production principle beyond the linear regime and towards steady state. Entropy 15, 2570–2584 (2013)
Martyushev, L.M.: Entropy and entropy production: old misconceptions and new breakthroughs. Entropy 15, 1152–1170 (2013)
Verhàs, J.: Gyarmati’s variational principle of dissipative processes. Entropy 16, 2362–2383 (2014)
Salamon, P., Hoffmann, K.H., Schubert, S., Berry, R.S., Andresen, B.: What conditions make minimum entropy production equivalent to maximum power production? J. Non Equilib. Thermodyn. 26, 73–83 (2001)
Polettini, M.: Macroscopic constraints for the minimum entropy production principle. Phys. Rev. E 84(051117), 1–9 (2011)
Beretta, G.P.: Modeling non-equilibrium dynamics of a discrete probability distribution: general rate equation for maximal entropy generation in a maximum-entropy landscape with time-dependent constraints. Entropy 10, 160–182 (2008)
Beretta, G.P.: Steepest entropy ascent model for far-nonequilibrium thermodynamics: unified implementation of the maximum entropy production principle. Phys. Rev. E 90, 1–14 (2014)
Uhlenbeck, G.E., Ornstein, L.S.: On the theory of the Brownian motion. Phys. Rev. 36, 823–841 (1930)
Mauri, R.: Non-Equilibrium Thermodynamics in Multiphase Flows. Springer, Heidelberg (2013)
Mauri, R., Leporini, D.: Violation of the fluctuation-dissipation theorem in confined driven colloids. Europhys. Lett. 76, 1022–1028 (2006)
Feynman, R.P., Hibbs, A.R.: Quantum Mechanics and Path Integrals. McGraw Hill, New York (1965)
Graham, R.: Path integral formulation of general diffusion processes. Z. Physik B 26, 281–290 (1977)
Wang, Q.A., El Kaabouchiu, A.: From random motion of Hamiltonian systems to Boltzmann’s H theorem and second law of thermodynamics: a pathway by path probability. Entropy 16, 885–894 (2014)
Gelfand, I.M., Yaglom, A.M.: Integration in function spaces and its application in quantum physics. J. Math. Phys. 1, 48–69 (1960)
Schulman, L.S.: Techniques and Applications of Path Integration, Chapter 9. Interscience, New York (1981)
Wiener, N.: The average of an analytic functional. Proc Nat. Acad. Sci. USA 7, 253–260; 294–298 (1921)
Wieger, F.W.: Introduction to Path Integral Methods in Physics and Polymer Science. World Scientific, Singapore (1986)
Martiouchev, L.M., Seleznev, V.D.: Maximum-entropy production principle as a criterion for the morphological-phase selection in the crystallization process. Dokl Phys 45, 129–131 (2000)
Molin, D., Mauri, R.: Spinodal decomposition of binary mixtures with composition-dependent heat conductivities. Chem. Eng. Sci. 63, 2402–2407 (2008)
Mauri, R., Haber, S.: Applications of the Wiener path integral for the diffusion of Brownian particles in shear flows. SIAM J. Appl. Math. 46, 49–55 (1986)
Foister, R.T., Van De Ven, T.G.M.: Diffusion of Brownian particles in shear flows. J. Fluid Mech. 96, 105–132 (1980)
Katayama, Y., Terauti, R.: Brownian motion of a single particle under shear flow. Eur. J. Phys. 17, 136–140 (1996)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mauri, R. The Principle of Minimal Resistance in Non-equilibrium Thermodynamics. Found Phys 46, 393–408 (2016). https://doi.org/10.1007/s10701-015-9969-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10701-015-9969-3