Abstract
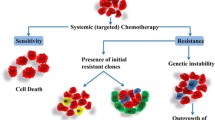
Although chemotherapy is one of the most common treatments for cancer, it can be only partially successful. Drug resistance is the main cause of the failure of chemotherapy. In this work, we present a mathematical model to study the impact of both intrinsic (preexisting) and acquired (induced by the drugs) resistances on chemotherapy effectiveness. Our simulations show that intrinsic resistance could be as dangerous as acquired resistance. In particular, our simulations suggest that tumors composed by even a small fraction of intrinsically resistant cells may lead to an unsuccessful therapy very quickly. Our results emphasize the importance of monitoring both intrinsic and acquired resistances during treatment in order to succeed and the importance of doing more experimental and genetic research in order to develop a pretreatment clinical test to avoid intrinsic resistance.




Similar content being viewed by others
References
Almendro V, Cheng YK, Randles A, Itzkovitz S, Marusyk A, Ametller E, Gonzalez-Farre X, Munoz M, Russnes HG, Helland A, Rye IH, Borresen-Dale AL, Maruyama R, van Oudenaarden A, Dowsett M, Jones RL, Reis-Filho J, Gascon P, Gonen M, Michor F, Polyak K (2014) Inference of tumor evolution during chemotherapy by computational modeling and in situ analysis of genetic and phenotypic cellular diversity. Cell Rep 6:514–527
Bertuzzi A, d’Onofrio A, Fasano A, Gandolfi A (2003) Regression and regrowth of tumour cords following single-dose anticancer treatment. Bull Math Biol 65:903–931
Casciari JJ, Sotirchos SV, Sutherland RM (1988) Glucose diffusivity in multicellular tumor spheroids. Cancer Res 48:3905–3909
Chaplain M, Matzavinos A (2006) Mathematical modeling of spatio-temporal phenomena in tumor immunology. In: Friedman A (ed) Tutorials in mathematical biosciences III: cell cycle, proliferation, and cancer. Springer, New York, pp 131–183
Coldman AJ, Goldie JH (1985) Role of mathematical modeling in protocol formulation in cancer chemotherapy. Cancer Treat Rep 69:1041–1048
de Pillis L, Fister KR, Gu W, Collins C, Daub M, Gross D, Moore J, Preskill B (2009) Mathematical model creation for cancer chemo-immunotherapy. Comput Math Methods Med 10:165–184
DeVita VT (1983) Progress in cancer management. Keynote address. Cancer 51:2401–2409
DeVita VT, Lawrence TS, Rosenberg SA, DePinho RA, Weinberg RA (2008) DeVita, Hellman, and Rosenberg’s cancer: principles and practice of oncology. Lippincott Williams & Wilkins, Philadelphia, USA
d’Onofrio A, Gandolfi A (2010) Resistance to antitumor chemotherapy due to bounded-noise-induced transitions. Phys Rev E 82(061):901
Drasdo D, Höhme S (2005) A single-cell-based model of tumor growth in vitro: monolayers and spheroids. Phys Biol 2:133–147
Fang J, Sullivan M, McCutchan TF (2004) The effects of glucose concentration on the reciprocal regulation of rRNA promoters in plasmodium falciparum. J Biol Chem 279:720–725
Foo J, Michor F (2010) Evolution of resistance to anti-cancer therapy during general dosing schedules. J Theor Biol 263:179–188
Freyer JP, Sutherland RM (1985) A reduction in the in situ rates of oxygen and glucose consumption of cells in EMT6/Ro spheroids during growth. J Cell Physiol 124:516–524
Frieboes HB, Edgerton ME, Fruehauf JP, Rose FRAJ, Worrall LK, Gatenby RA, Ferrari M, Cristini V (2009) Prediction of drug response in breast cancer using integrative experimental/computational modeling. Cancer Res 69:4484–4492
Garner AL, Lau YY, Jackson TL, Uhler MD, Jordan DW, Gilgenbach RM (2005) Incorporating spatial dependence into a multicellular tumor spheroid growth model. J Appl Phys 98:1–8
Gerlinger M, Rowan AJ, Horswell S, Larkin J, Endesfelder D, Gronroos E, Martinez P, Matthews N, Stewart A, Tarpey P, Varela I, Phillimore B, Begum S, McDonald NQ, Butler A, Jones D, Raine K, Latimer C, Santos CR, Nohadani M, Eklund AC, Spencer-Dene B, Clark G, Pickering L, Stamp G, Gore M, Szallasi Z, Downward J, Futreal PA, Swanton C (2012) Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. New Engl J Med 366:883–892
Goldie JH, Coldman AJ (2009) Drug resistance in cancer: models and mechanisms. Cambridge University Press, Cambridge, UK
Jackson TL (2003) Intracellular accumulation and mechanism of action of doxorubicin in a spatio-temporal tumor model. J Theor Biol 220:201–213
Jiang Y, Pjesivac-Grbovic J, Cantrell C, Freyer JP (2005) A multiscale model for avascular tumor growth. Biophys J 89:3884–3894
Johnstone RW, Ruefli AA, Lowe SW (2002) Apoptosis: a link between cancer genetics and chemotherapy. Cell 108:153–164
Kansal AR, Torquato S, Chiocca EA, Deisboeck TS (2000) Emergence of a subpopulation in a computational model of tumor growth. J Teor Biol 207:431–441
Kole AC, Plaat BEC, Hoekstra HJ, Vaalburg W, Molenaar WM (1999) FDG and L-[1-11C]-tyrosine imaging of soft-tissue tumors before and after therapy. J Nucl Med 40:381–386
Lavi O, Gottesman MM, Levy D (2012) The dynamics of drug resistance: a mathematical perspective. Drug Resist Update 15:90–97
Lecca P, Morpurgo D (2012) Modelling non-homogeneous stochastic reaction–diffusion systems: the case study of gemcitabine-treated non-small cell lung cancer growth. BMC Bioinform. doi:10.1186/1471-2105-13-S14-S14
Lippert TH, Ruoff HJ, Volm M (2008) Resistance in malignant tumors: Can resistance assays optimize cytostatic chemotherapy? Pharmacology 81:196–203
Lippert TH, Ruoff H, Volm M (2011) Current status of methods to assess cancer drug resistance. Int J Med Sci 8:245–253
McKinnell RG, Parchment RE, Perantoni AO, Pierce GB, Damjanov I (2006) The biological basis of cancer. Cambridge University Press, New York, USA
Menchón SA, Condat CA (2008) Cancer growth: predictions of a realistic model. Phys Rev E 78:022901
Menchón SA, Condat CA (2009) Modeling tumor cell shedding. Eur Biophys J 38:479–485
Menchón SA, Condat CA (2011) Quiescent cells: a natural way to resist chemotherapy. Phys A 390:3354–3361
Murray JM, Coldman AJ (2003) The effect of heterogeneity on optimal regimens in cancer chemotherapy. Math Biosci 185:73–87
Norris ES, King JR, Byrne HM (2006) Modelling the response of spatially structured tumours to chemotherapy: drug kinetics. Math Comput Model 43:820–837
Panetta JC (1998) A mathematical model of drug resistance: heterogeneous tumors. Math Biosci 147:41–61
Priestman T (2008) Cancer chemotherapy in clinical practice. Springer-Verlag, London
Raguz S, Yagüe E (2008) Resistance to chemotherapy: new treatments and novel insights into an old problem. Br J Cancer 99:387–391
Scalerandi M, Romano A, Pescarmona GP, Delsanto PP, Condat CA (1999) Nutrient competition as a determinant for cancer growth. Phys Rev E 59(2):2206–2217
Silva AS, Gatenby RA (2010) A theoretical quantitative model for evolution of cancer chemotherapy resistance. Biol Direct 5:25
Stein WD, Figg WD, Dahut W, Stein AD, Hoshen MB, Price D, Bates SE, Fojo T (2008) Tumor growth rates derived from data for patients in a clinical trial correlate strongly with patient survival: a novel strategy for evaluation of clinical trial data. Oncologist 13:1046–1054
Swan GW (1990) Role of optimal control theory in cancer chemotherapy. Math Biosci 101:237–284
Swanson KR, Bridge C, Murray JD, Alvord EC (2003) Virtual and real brain tumors: using mathematical modeling to quantify glioma growth and invasion. J Neurol Sci 216(1):1–10
Swierniak A, Kimmel M, Smieja J (2009) Mathematical modeling as a tool for planning anticancer therapy. Eur J Pharmacol 625:108–121
Acknowledgments
This work was partially supported by SeCyT-UNC (project 3072011-0100436) and CONICET (PIP 112-200801-00772) (Argentina). I would like to express my gratitude to Prof. Carlos Condat for his guidance, helpful advice and inspiring discussions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Menchón, S.A. The Effect of Intrinsic and Acquired Resistances on Chemotherapy Effectiveness. Acta Biotheor 63, 113–127 (2015). https://doi.org/10.1007/s10441-015-9248-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10441-015-9248-x