Abstract
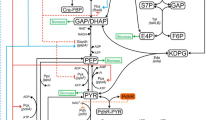
Threonine is an essential amino acid for mammals and birds and an adequate supply is necessary for growth and maintenance. Its production has become the aim of metabolic bioengineering and genetic manipulations. We propose in this paper a rational approach for increasing threonine production in anE. coli strain based on metabolic control theory. We have derived a way to measure the control coefficients of threonine pathwayin vivo. The method consists in modelling the results of presteady-state experiments. Thein vivo concentrations and activities of the enzymes can then be measured and introduced into the model, so that thein vivo steady-state of the pathway can be evaluated. With such a model it is possible to calculate the theoretical values of the control coefficients of the threonine synthesis fluxin vivo.
Similar content being viewed by others
References
Black, S. and N.G. Wright (1955a). β-aspartokinase and β-aspartylphosphate. J. Biol. Chem. 213: 27–38.
Black, S. and N.G. Wright (1955b). Asparticβ-semialdehyde dehydrogenase and asparticβ-semialdehyde. J. Biol. Chem. 213: 39–50.
Cohen, G.N. (1967). Le Métabolisme Cellulaire et sa Régulation. Paris, Hermann.
Cremer, J., L. Eggeling and H. Salm (1991). Control of the lysine biosynthesis sequence inCorynebacterium glutamicum as analyzed by overexpression of the individual corresponding genes. Appl. Environ. Microbiol. 57: 1746–1752.
Fell, D.A. and K. Snell (1988). Control analysis of mammalian serine biosynthesis. Biochem. J. 256: 97–101.
Hegeman G.D. and G.N. Cohen (1968). Aspartic semialdehyde dehydrogenase. In: L.C. Kuo and J.A. Shafer, eds., Methods in enzymology 17, p. 708–713.
Heinrich, R. and T.A. Rapoport (1974). A linear steady-state treatment of enzymatic chains. General properties, Control and Effector Strengt. Eur. J. Biochem. 42: 89–95.
Henri, V. (1903). Lois générales de l'action des disatases. Paris, Thèse.
Houssin, C., N. Eynard, E. Shechter and A. Ghazi (1991). Effect of osmotic pressure on membrane energy-linked functions inEsherichia coli. Biochim Biophys. Acta 1056: 76–84.
Ishida, M., K. Sato, K. Hashiguchi, H. Ito, H. Enei and S. Nakamori (1993). High fermentative production of L-threonine from acetate by aBrevibacterium flavum stabilized strain transformed with a recombinant plasmid carrying theEscherichia coli thr operon. Blosci. Biotech. Biochem. 57: 1755–1756.
Joseph, M.H. and C.A. Marsden (1986). Amino acids and small peptides. In: Lim, C.K. ed., HPLC of Small Molecules. A Pratical Approach, chp.2, 13–28. IRL press.
Kacser, H. and L. Acerenza (1993). A universal method for achieving increases in metabolite production. Eur. J. Biochem. 216: 361–367.
Kacser, H. and J.A. Burns (1973). The control of flux. In: Davies D.D. ed., Rate Control of Biological Processes, 65–104. Cambridge University Press.
Mazat, J.-P. and J.-C. Patte (1976). Lysine-sensitive aspartokinase ofE. coli K12. Synergy and auto-synergy in an allosteric V-system. Biochemistry 15: 4053–4058.
Neidhardt, F.C. (1987).Escherichia coli andSalmonella typhimurium. Cellular and molecular biology. American Society for Microbiology. Washington, D.C.
Niederberger, P., R. Prasad, G. Miozzari and H. Kacser (1992). A strategy for increasing anin vivo flux by genetic manipulations. The tryptophan system of yeast. Biochem. J. 287: 473–479.
Patte J.-C. and G.N. Cohen (1964). Interactions coopératives effecteur-effecteur chez deux enzymes allostériques inhibées de manière non-compétitive. C.R.Acad. Sc. Paris 259: 1255–1258.
Raïs, B. and J.-P. Mazat (1995). Contrôle de la chaîne de biosynthèse de la thréonine chezE. coli: application en biotechnologies. Acta Biotheoretica 43: 143–153.
Szczesiul, M. and D.E. Wampler (1976). Regulation of a metabolic system in vitro: Synthesis of threonine from aspartate. Biochem. 15, 10: 2236–2244.
Varma, A., B.W. Boesch and B.O. Palsson (1993). Biochemical production capabilities ofEscherichia coli. Biotechnol. Bioeng. 42: 57–73.
Wampler, D.E. and E.W. Westead (1968). Two aspartokinases fromE. coli. Nature of the inhibition and molecular changes accompanying reversible inactivation. Biochem. 7. 5 1661–1671.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Raïs, B., Chassagnole, C. & Mazat, J.P. Control of threonine pathway in E. coli. application to biotechnologies. Acta Biotheor 43, 285–297 (1995). https://doi.org/10.1007/BF00713554
Issue Date:
DOI: https://doi.org/10.1007/BF00713554