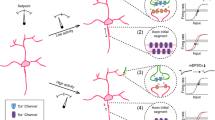
Abstract
The emergence of functional maturity in the brain relies upon the interplay between form and function during its developmental history. This interaction continues throughout life and changes in neuronal function lead to changes in diverse structural scales in the brain. Both regulatory genes, which define compartments in the nervous system, and activity-dependent processes cooperate to determine neuronal phenotype and tissue structure. The influence of electrical activity as a regulator of early developmental events such as proliferation and migration is being considered. Spontaneous neuronal activity may influence early axonal and dendritic arbor formation and activity blockade alters branch density both for axon arbors and dendrites. Although large-scale changes in axon morphology may occur until late stages of development, the remodeling of axon and dendrite morphology in terms of their terminal arborization area is less pronounced than initially thought. Electrical (or ‘neural’) activity is important for synapse stabilization and circuit formation and sensory experience performs a refinement of neuronal shape. This fine-tuning appears to be a dynamical process sustained into adulthood, with smaller scale changes occurring mainly at the dendritic spine level. These subcellular compartments are now believed to restrict biochemical changes in dendrites to particular synapses during (or ‘undergoing’) synaptic plasticity. Events at dendritic spines underlie alterations in the morphology of individual neurons that will ultimately affect the function of complex neuronal networks.
Similar content being viewed by others
References
Allendoerfer, K. L. and Shatz, C. J., 1994: The subplate, a transient neocortical structure: Its role in the development of connections between thalamus and cortex, Annu. Rev. Neurosci. 17, 185-218.
Altman, J., 1971: Coated vesicles and synaptogenesis. A developmental study in the cerebellar cortex of the rat, Brain Res. 30, 311-322.
Antonini, A. and Stryker, M. P., 1993: Development of individual geniculocortical arbors in cat striate cortex and effects of binocular impulse blockade, J. Neurosci. 13, 3549-3573.
Barron, D. H., 1943: The early development of the motor cells and columns in the spinal cord of the sheep, J. Comp. Neurol. 78, 1-26.
Bedlack, R., Wei, M., Fox, S., Gross, E. and Loew, L., 1994: Distict electrical potentials in soma and neurite membranes, Neuron 13, 1187-1193.
Bhide, P. G. and Frost, D. O., 1991: Stages of growth of hamster retinofugal axons: Implications for developing axonal pathways with multiple targets, J. Neurosci. 11, 485-504.
Bliss, T. and Lomo T., 1973: Long-lasting potentiation of synaptic transmission in the dendate area of anesthetized rabbit following stimulation of the perforant path, J. Physiol. 232, 331-356.
Bliss, T. V. P. and Collingridge, G. L., 1993: A synaptic model of memory Long-term potentiation in the hippocampus, Nature 361, 31-39.
Bode-Greual, K. and Singer, W., 1989: The development of N-methyl-D-aspartate receptors in cat visual cortex, Dev. Brain Res. 46, 197-204.
Borrell, V. and Callaway, E. M., 2002: Reorganization of exuberant axon arbors contributes to the development of laminar specificity in ferret visual cortex. J. Neurosci. 22, 6682-6695.
Boyer, C., Schikorski, T. and Stevens, C. F., 1998: Comparison of hippocampal dendritic spines in culture and in brain, J. Neurosci. 18, 5294-300.
Brandes, J. S., 1971: Dendritic branching patterns in lateral geniculate nucleus following deafferentation, Exp. Neurol. 31, 444-450.
Bresler, T., Ramati, Y., Zamorano, P. L., Zhai, R., Garner, C.C. and Ziv, N. E., 2001: The dynamics of SAP90PSD-95 recruitment to new synaptic junctions, Mol. Cell. Neurosci. 18, 149-167.
Brose, K. and Tessier-Lavigne, M., 2000: Slit proteins: Key regulators of axon guidance, axonal branching, and cell migration. Curr. Opin. Neurobiol. 10, 95-102.
Brostom, C. and Brostom, M., 1990: Calcium-dependent regulation of protein synthesis in intact mammalian cells, Annu. Rev. Physiol. 52, 577-590.
Brown, T., Chapman, P., Kairiss, E. and Keenan, C., 1988: Long-term potentiation, Science 242, 724-728.
Brunso-Bechtold, J. K. and Vinsant, S. L., 1990: An ultrastructural and morphometric study of the effect of removal of retinal input on the development of the dorsal lateral geniculate nucleus, J. Comp. Neurol. 301, 585-603.
Buonanno, A. and Fields, D. R., 1999: Gene regulation by patterned electrical activity during neural and skeletal muscle development, Curr. Opin. Neurobiol. 9, 110-120.
Buonomano, D. V. and Merzenich, M. M., 1998: Cortical plasticity: From synapses to maps, Annu. Rev. Neurosci. 21, 149-186.
Butler, A. B. and Hodos, W., 1996: Comparative Vertebrate Neuroanatomy, Wiley-Liss, New York.
Callaway, E. M. and Katz, L. C., 1990: Emergence and refinement of clustered horizontal connections in cat striate cortex, J. Neurosci. 10, 1134-1153.
Campbell, D. S., Regan, A. G., Lopez, J. S., Tannahill, D., Harris, W. A., Holt, C. E. 2001: Semaphorin 3A elicits stage-dependent collapse, turning, and branching in Xenopus retinal growth cones, J. Neurosci. 21(21): 8538-8547.
Castellani, V., Yue, Y., Gao, P. P., Zhou, R., Bolz, J., 1998: Dual action of a ligand for Eph receptor tyrosine kinases on specific populations of axons during the development of cortical circuits, J. Neurosci. 18, 4663-4672.
Chalupa, L. M. and Williams, R.W., 1984: Organization of the cat's lateral geniculate nucleus following interruption of prenatal binocular competition, Human Neurobiol 3, 103-108.
Chiaia, N. L., Bauer, W. R., Bennett-Clarke, C. A. and Rhoades, R. W., 1992: Postnatal blockade of cortical activity by tetrodotoxin does not disrupt the formation of vibrissae-related patterns in the rat's somatosensory cortex, Dev. Brain Res. 66, 244-250.
Cline, H. T. and Constantine-Paton, M., 1989: NMDA receptor antagonists disrupt the retinotectal topographic map, Neuron 3, 413-426.
Cline, H. T. and Constantine-Paton, M., 1990: NMDA receptor agonist and antagonists alter retinal ganglion cell arbor structure in the developing frog retinotectal projection, J. Neurosci. 10, 1197-1216.
Cline, H. T., Debski, E. and Constantine-Paton, M., 1987: NMDA receptor antagonist desegregates eye-specific stripes, Proc. Natl. Acad. Sci. USA 84, 4342-4345.
]Cooper, N. and Steindler, D., 1986: Lectins demarcate the barrel subfield in the somatosensory cortex of the early postnatal mouse, J. Comp. Neurol. 249, 157-169.
Coss, R. G. and Perkel, D. H., 1985: The function of dendritic spines: A review of theoretical issues, Behav Neural Biol. 44, 151-185.
Cotman, C. W., Matthews, D. A., Taylor, D., and Lynch, G., 1973: Synaptic rearrangement in the dentate gyrus: Histochemical evidence of adjustments after lesions in immature and adult rats. Proc. Natl. Acad. Sci. USA 70, 3473-3477.
Cotman, C., Monaghan, D. and Ganong, A., 1988: Excitatory amino acid neurotransmission: NMDA receptors and Hebb-type synaptic plasticity, Ann. Rev. Neurosci. 11, 61-80.
Crain, B., Cotman, C., Taylor, D. and Lynch, G., 1973: Aquantitative electron microscopic study of synaptogenesis in the dentate gyrus of the rat. Brain Res. 63, 195-204.
Crowley, J. C. and Katz, L. C., 2000: Early development of ocular dominance columns. Science 290, 1321-1324.
Dailey, M. E. and Smith, S. J., 1996: The dynamics of dendritic structure in developing hippocampal slices. J. Neurosci. 16, 2983-2994.
Dalva, M. B., Ghosh, A. and Shatz, C. J., 1994: Independent control of dendritic and axonal form in the developing lateral geniculate nucleus, J. Neurosci. 14, 3588-3602.
Darwin, C., 1994: The Origin of Species by Means of Natural Selection, Senate, London.
Davidson, E. H., 2001. Genomic regulatory systems: Development and evolution, Academic Press, New York.
Dehay, C., Giroud, P., Berland, M., Smart, I. and Kennedy, H., 1993: Modulation of the cell cycle contributes to the parcellation of the primate visual cortex, Nature 366, 464-466.
Dehay, C., Savatier, P., Cortay, V. and Kennedy, H., 2001: Cell-cycle kinetics of neocortical precursors are influenced by embryonic thalamic axons, J. Neurosci. 21, 201-214.
Deitch, J. S. and Rubel, E.W., 1984: Afferent influences on brain stem auditory nuclei of the chicken: The course and specificity of dendritic atrophy following deafferentation, J. Comp. Neurol. 229, 66-79.
Deller, T., Mundel, P. and Frostscher, M., 2000: Potential role of synaptopodin in spine motility by coupling actin to the spine apparatus, Hippocampus 10, 569-581.
Desmond, N. and Levy, W., 1985: Granule cell dendritic spines density in the rat hippocampus varies with spine shape and location, Neurosci. Lett. 54, 219-224.
Douglas, R. J. and Martin K. A. C., 1990. Neocortex. In: The synaptic organization of the brain, in G. M. Shepherd (ed), Oxford University Press, Oxford, Chap. 12.
Drake, C. T., Milner, T. A. and Patterson, S. L., 1999: Ultrastructural localization of full-length trkB immunoreactivity in rat hippocampus suggests multiple roles in modulating activity-dependent synaptic plasticity, J. Neurosci. 19, 8009-8026.
Drakew, A., Frostscher, M., and Heimrich, B., 1999: Blockade of neuronal activity alters spine maturation of dentate granule cells but not their dendritic arborization, Neuroscience 94, 767-774.
Edelman, G. M., 1987: Neural Darwinism. Basic Books, New York.
El-Hani, C. N. and Emmeche, C., 2000: On some theoretical grounds for an organism-centred biology: Property emergence, supervenience, and downward causation, Theory Biosci. 119, 234-275.
Engert, F. and Bonhoeffer, T., 1999: Dendritic spine changes associated with hippocampal long-term synaptic plasticity. Nature 399, 66-70.
Erzurumlu, R. and Kind, P., 2001: Neural activity: Sculptor of barrels in the neocortex. TINS 24, 589-595.
Fazeli, A., Dickinson, S. L., Hermiston, M. L., Tighe, R.V., Steen, R. G., Small, C. G., Stoeckli, E. T., Keino-Masu, K., Masu, M., Rayburn, H., Simons, J., Bronson, R. T., Gordon, J. I., Tessier-Lavigne, M. and Weinberg, R. A., 1997: Phenotype of mice lacking functional deleted in colorectal cancer (DCC) gene. Nature 386, 796-804.
Finlay, B. L., Wikler, K. C. and Sengelaub, D. R., 1987: Regressive events in brain development and scenarios for vertebrate brain evolution. Brain Behav. Evol. 30, 102-117.
Fischer, M., Kaech, S., Knutti, D. and Matus, A, 1998: Rapid actin-based plasticity in dendritic spines, Neuron 20, 847-854.
Fischer, M., Kaech, S., Wagner, U., Brinkhaus, H. and Matus, A., 2000: Glutamate receptors regulate actin-based plasticity in dendritic spines, Nat. Neurosci. 3, 887-894.
Frank, E., 1987: The influence of neuronal activity on patterns of synaptic connections, TINS 10, 188-190.
Freiwald, W. A., Kreiter, A. K. and Singer,W., 2001: Synchronization and assembly formation in the visual cortex, Prog. Brain. Res. 130, 111-140.
Friedman, H. V., Bresler, T., Garner, C. C. and Ziv, N. E., 2000: Assembly of new individual excitatory synapses: Time course and temporal order of synaptic molecule recruitment, Neuron 27, 3-5.
Frisen, J., 2002: Stem cell plasticity? Neuron 35, 415-418.
Fròes, M. M. and Menezes, J. R. L., 2002: Coupled heterocellular arrays in the brain. Neurochem. Int. 41, 364-375.
Garraghty, P. E., Sur, M., Weller, R. and Sherman, S. M., 1986: Morphology of retinogeniculate X and Y axon arbors in monocularly enucleated cats, J. Comp. Neurol. 251, 198-215.
Geinisman, Y., Morrell, F. and Toledo-Morrell, L., 1989: Perforated synapses on double-headed dendritic spines: A possible structural substrate of synaptic plasticity, Brain Res. 480, 326-329.
Gentschev, T. and Sotelo, C., 1973: Degenerative patterns in the ventral cochlear nucleus of the rat after primary deafferentation. An ultrstructural study, Brain Res. 62, 37-60.
Ghosh, A., Antonini, A., McConnell, S. and Shatz, C. J., 1990: Requirement for subplate neurons in the formation of thalamocortical connections, Nature 347, 170-181.
Ghosh, A. and Shatz, C., 1992: Pathfinding and target selection by developing geniculocortical axons, J. Neurosci. 12, 39-55.
Ghosh, A. and Shatz, C., 1993: A role for subplate neurons in the patterning of connections from thalamus to neocortex, Development 117, 1031-1047.
Ghosh, A. and Shatz, C., 1994: Segregation of geniculocortical afferents during the critical period: A role for subplate neurons, J. Neurosci. 14, 3862-3880.
Goda, Y., 2002: Cadherins communicate structural plasticity of presynaptic and postsynaptic terminals, Neuron 35, 1-3.
Greenough, W. T. and Volkmar, F. R., 1973: Pattern of dendritic branching in occipital cortex of rats reared in complex environments, Exp. Neurol. 40, 491-504.
Gu, X. and Spitzer, N. C., 1995: Distinct aspects of neuronal differentiation encoded by frequency of spontaneus Ca2C transients. Nature 375, 784-787.
Guillery, R. and Kaas, J., 1974: The effects of monocular lid suture upon the development of the lateral geniculate nucleus in squirrels (Sciureus carolinensis), J. Comp. Neurol. 154, 433-441.
Guillery, R. W., 1972: Binocular competition in the control of geniculate cell growth, J. Comp. Neurol. 144, 117-130.
Guillery, R.W., 1973: Quantitative studies of transneuronal atrophy in the dorsal lateral geniculate nucleus of cats and kittens, J. Comp. Neurol. 149, 423-438.
Guthrie, P., Segal, M. E. and Kater, S., 1991: Independent regulation of calcium revealed by imaging dendritic spines, Nature 354, 76-79.
Hahm, J. O., Langdon, R. B. and Sur, M., 1991: Disruption of retinogeniculate afferent segregation by antagonists to NMDA receptors, Nature 351, 568-570.
Hanganu, I. L., Kilb, W. and Luhmann, H. J., 2002: Functional synaptic projections onto subplate neurons in neonatal rat somatosensory cortex. J. Neuronsci. 22, 7165-7176.
Harris, K. and Stevens, J., 1988: Dendritic spines of rat cerebellar Purkinje cells: Serial electron microscopy with reference to their biophysical characteristics, J. Neurosci. 8, 4455-4469.
Harris, K. and Stevens, J., 1989: Dendritic spines of CA1 pyramidal cells in the rat hippocampus: Serial electron microscopy with reference to their biophysical characteristics, J. Neurosci. 9, 2982-2997.
Harris, K. M., 1999: Structure, development ans plasticity of dendritic spines, Curr. Opin. Neurobiol. 9, 343-348.
Hatten, M. and Mason, C., 1986: Neuron-astroglia interactions in vitro and in vivo. Trends Neurosci. 9, 168-174.
Haydar, T. F., Wang, F., Schwartz, M. L. and Rakic, P., 2000: Differential modulation of proliferation in the neocortical ventricular and subventricular zones, J. Neurosci. 20, 5764-5774
Hebb, D., 1949: Organization of Behavior, Wiley, New York.
Hedin-Pereira, C., Lent, R. and Jhaveri, S., 1999: Morphogenesis of callosal arbors in the parietal cortex of hamsters, Cereb. Cortex, 9, 50-64.
Hedin-Pereira, C., Uziel, D. and Lent, R., 1992: Bicommissural neurons in the cerebral cortex of developing hamsters, NeuroReport 3, 873-876.
Herrmann, K., Antonini, A. and Shatz, C., 1994: Ultrastructural evidence for synaptic interactions between thalamocortical axons and subplate neurons. Eur. J. Neurosci. 6, 1729-1742.
Herrmann, K., Wong, R. and Shatz, C., 1992: Effects of intraocular activity blockade on the morphology of developing dLGN neurons in the cat, in P. Bagnoli and W. Hodos (eds.), The Changing Visual System, Plenum Press, New York.
Hinds, J. and Hinds, P., 1976: Synapse formation in the mouse olfatory bulb. II. Morphogenesis, J. Comp. Neurol. 169, 41-62.
Jhaveri, S., Edwards, M. A. and Schneider, G. E., 1991: Initial stages of retinofugal axon development in the hamster: Evidence for two distinct modes of growth, Exp. Brain Res. 87, 371-382.
Katz, L. C., 1991: Specificity in the development of vertical connections in the striate cortex, Eur. J. Neurosci. 3, 1-9.
Katz, L. C. and Callaway, E. M, 1991: Emergence and refinement of local circuits in cat striate cortex, in Development of the Visual System, MIT Press, Cambridge, MA, pp. 197-215.
Katz, L. C. and Crowley, J. C, 2002: Development of cortical circuits: Lessons from ocular dominance columns. Nat. Rev. Neurosci. 3, 34-42.
Katz, L. C. and Shatz, C. J., 1996. Synaptic activity and the construction of cortical circuits. Science, 274, 1133-1138.
Kennedy, M., 1989: Regulation of neuronal function by calcium, Trends Neurosci. 12, 417-420.
Koch, C. and Poggio, T., 1983: Electrical properties of dendritic spines, Trends Neurosci. 6, 80-83.
Koch, C. and Zador, A., 1993: The function of dendritic spines: Devices subserving biochemical rather than electrical compartmentalization, J. Neurosci. 13, 413-422.
Kojima, M., Takei, N., Numakawa, T., Ishikawa, Y., Susuki, S., Matsumoto, T., Katoh-Semba, R., Nawa, H. and Hatanaka, H., 2001: Biological characterization and optical imaging of brain-derived neurotrophic factor-green fluorescent protein suggest an activity-dependent local release of brain-derived neurotrophic factor in neurites of cultured hippocampal neurons, J. Neurosci. Res. 64, 1-10.
Komatsu, Y., Fujii, K., Maeda, J., Sakaguch, I. H. and Toyama, K., 1988: Long-term potentiation of synaptic transmission in kitten visual cortex, J. Neurophysiol. 59, 124-141.
Korkotian, E. and Segal, M., 2001: Regulation of dendritic spine motility in cultured hippocampal neurons, J. Neurosci. 21, 6115-6124.
Kozloski, J., Hamzei-Sichani, F. and Yuste, R., 2001: Stereotyped position of local synaptic targets in neocortex. Science 293, 868-872.
Landis, D. M., 1987: Initial junctions between developing parallel fibers and Purkinje cells are different from mature synaptic junctions, J. Comp. Neurol. 260, 513-525.
Lent, R., Hedin-Pereira, C., Menezes, J. R. L. and Jhaveri, S., 1990: Neurogenesis and development of callosal and intracortical connections in the hamster, Neuroscience 38, 21-37.
LeVay, S., Stryker, M. P. and Shatz, C. J., 1978: Ocular dominance columns and their development in layer IV of the cat's visual cortex: A quantitative study, J. Comp. Neurol. 179, 223-244.
Leventhal, A., Schall, J. and Ault, S., 1988: Extrinsic determinants of retinal ganglion cell structure in the cat, J. Neurosci. 8, 2028-2038.
Linke, R., Soriano, E. and Frotscher, M., 1994: Transient dendritic appendages on differentiating septohippocampal neurons are not sites of synaptogenesis, Dev. Brain Res. 83, 67-78.
Lo, Y.-J. and Poo, M.-M., 1994: Heterosynaptic supression of developing neuromuscular synapses in culture, J. Neurosci. 14, 4684-4693.
Luskin, M. B. and Shatz, C. J., 1985: Neurogenesis of the cat's primary visual cortex, J. Comp. Neurol. 242, 611-631.
Maletic-Savatic, M., Malinow, R. and Svoboda, K., 1999: Rapid dendritic morphogenesis in CA1 hippocampal dendrites induced by synaptic activity, Science 283, 1923-1927.
Mariani, J., Crepel, F., Mikoshiba, K., Changeux, J. and Sotelo, C., 1977: Anatomical, physiological and biochemical studies of the cerebellum from reeler mutant mouse, Philos. Trans. R. Soc. Lond. B Biol. Sci. 281, 1-28.
Marin, O. and Rubenstein, J. L., 2001: A long, remarkable journey: Tangential migration in the telencephalon. Nat. Rev. Neurosci. 2, 780-790.
Marrs, G. S., Green, S. H. and Dailey, M. E., 2001: Rapid formation and remodelling of postsynaptic densities in developing dendrites, Nature Neurosci 4, 1006-1113.
Mattson, M., 1988: Neurotransmitters in the regulation of neuronal cytoarchitecture, Brain Res. Rev. 13, 179-212.
Matus, A., Brinkhaus, H. and Wagner, U., 2000: Actin dynamics in dendritic spines: A form of regulated plasticity at excitatory synapses, Hippocampus 10, 555-560.
Mayr, E., 1998: O desenvolvimento do pensamento biológico, Universidade de Brasília, Brasília.
Meister, M., Wong, R. O. L., Baylor, D. A. and Shatz, C. J., 1991: Synchronous bursts of action potentials in ganglion cells of developing mammalian retina, Science 252, 939-943.
Mugnaini, E., 1969: Maturation of nerve cell populations and establishment of synaptic connections in the cerebellar cortex of the chick, in R. Llin´as (ed.), Neurobiology of Cerebellar Evolution and Development, American Medical Association, Chicago, pp. 749-782
Muller, D., Toni, N. and Buchs, P. A., 2000: Spine changes associated with long-term potentiation, Hippocampus 10, 594-604.
Muller, W. and Connor, J., 1991: Dendritic spines as individual neuronal compartments for synaptic Ca2C responses, Nature 354, 73-76.
Murase, S., Mosser, E. and Schuman, E. M., 2002: Depolarization drives beta-catenin into neuronal spines promoting changes in synaptic structure and function, Neuron 35, 91-105.
Murphey, R., Mendenhall B., Palka, J. and Edwards, J., 1975: Deafferentation slows the growth of specific dendrites of identified giant interneurons, J. Comp. Neurol. 159, 407-418.
Nicholson, C., 1980: Dynamics of the brain cell microenvironment, Neurosci. Res. Prog. Bull. 18, 177-322.
Nowak, L., Bregestovski, P., Ascher, P., Herbert, A. and Prochiantz, A., 1984: Magnesium gates glutamate-activated channels in mouse central neurons, Nature 307, 462-465.
Okabe, S., Miwa, A. and Okado, H., 2001: Spine formation and correlated assembly of presynaptic and postsynaptic molecules, J. Neurosci. 21, 6105-6114.
O'Leary, D. D. M. and Terashima, T., 1988: Cortical axons branch to multiple subcortical targets by interstitial budding: Implications for target recognition and “waiting periods', ” Neuron 1, 901-910.
O'Leary, D.D. and Nakagawa,Y., 2002: Patterning centers, regulatory genes and extrinsic mechanisms controlling arealization of the neocortex, Curr. Opin. Neurobiol. 12, 14-25.
Panchision, D. M. and McKay, R. D., 2002: The control of neural stem cells by morphogenic signals, Curr. Opin. Gen. Dev. 12, 478-487.
Perkel, D. H. and Perkel, D. J., 1985: Dendritic spines: Role of active membrane in modulating synaptic efficacy, Brain Res. 325, 331-335.
Perry, V. H. and Linden, R., 1982: Evidence for dendritic competition in the developing retina, Nature 297, 683-685.
Perry, V. H. and Maffei, L., 1988: Dendritic competition: Competition for what? Dev. Brain Res. 41, 195-208.
Purves, D. and Lichtman, J.W., 1985: Geometrical differences among homologous neurons in mammals, Science 228, 298-302.
Rabacchi S., Baily, Y., Delhaye-Bouchaud, N. and Mariani, J., 1992: Involvement of the N-methyl-D-aspartate (NMDA) receptor in synapse elimination during cerebellar development. Science 256, 1823-1825.
Rajan, I. and Cline, H. T., 1998: Glutamate receptor activity is required for normal development of tectal cell dendrites in vivo. J. Neurosci. 18, 7836-7846.
Rajan, I., Witte, S., Cline, H. T., 1999: NMDA receptor activity stabilizes presynaptic retinotectal axons and postsynaptic optic tectal cell dendrites in vivo. J. Neurobiol. 38, 357-368.
Rakic, P., 1976: Prenatal genesis of connections subserving ocular dominance in the rhesus monkey, Nature 261, 467-471.
Rakic, P., Bourgeois, J., Eckenhoff, M., Zecevic, N. and Goldman-Rakic, S., 1986: Concurrent overproduction of synapses in diverse regions of the primate cerebral cortex, Science 232, 232-235.
Rall,W., 1978: Dendritic spines and synaptic potency, in Studies in Neurophysiology, Porter, Cambridge, pp. 203-209.
Ramoa, A. and McCormick, D., 1994: Developmental changes in eletrophysiological properties of LGNd neurons during reorganization of retinogeniculate connections, J. Neurosci. 14, 2089-2097.
Ramon y Cajal, 1893: La rétine des vertebrés, La Cellule 9, 119-258.
Ramon y Cajal, S., 1911: Histologie du systeme nerveux chez l'homme et chez les vertebras, vol, 2 L. Azoulay (trans.) Paris: Maloina. Republished in 1955. marked: Instituto Ramony cage.
Redies, C. and Puelles, L., 2001: Modularity in vertebrate brain development and evolution, BioEssays 23, 1100-1111.
Redmond, L., Kashani, A. H. and Ghosh, A., 2002: Calcium regulation of dendritic growth via CaM Kinase IV and CREB-mediated transcription, Neuron 34, 999-1010.
Reese, B. E., 1986: The topography of expanded uncrossed retinal projections following neonatal enucleation of one eye: Differing effects in dorsal lateral geniculate nucleus and superior colliculus, J. Comp. Neurol. 250, 8-32.
Riccio, R. V. and Matthews, M., 1987: Effects of intraocular tetrotoxin on the postnatal development of the dorsal lateral geniculate nucleus of the rat: A Golgi analysis, J. Neurosci. Res. 17, 440-451.
Rocha, M., 1995: Dendritic development of neurons of the ferret lateral geniculate nucleus: Role of NMDA receptors, PhD dissertation, Universidade Federal do Rio de Janeiro, Brazil.
Rocha, M. and Sur, M., 1995: Rapid acquisition of dendritic spines by visual thalamic neurons after blockade of N-methyl-D-aspartate receptors, Proc. Natl. Acad. Sci. USA 92, 8026-8030.
Roe, A., Pallas, S. L., Hahm, J.-O. and Sur, M., 1990: A map of visual space induced in primary auditory cortex, Science 250, 818-820.
Ruiz i Atalba, A., Gitton, G. and Dahmane, N. 2001: Embryonic regionalization of the neocortex. Mech. Dev. 107, 1-14.
Rutishauser, U. and Landmesser, L., 1991: Polysialic acid on the surface of axons regulates patterns of normal and activity-dependent innervation, Trends Neurosci. 14, 528-532.
Schuman, E. M. and Madison, D. V., 1991: A requirement for the intercellular messenger nitric oxide in long-term potentiation, Science 254, 1503-1506.
Serafini, T., Colamarino, S. A., Leonardo, E. D., Wang, H., Beddington, R., Skarnes, W. C. and Tessier-Lavigne, M., 1996: Netrin-1 is required for commissural axon guidance in the developing vertebrate nervous system. Cell 87, 1001-1014.
Shi, S. H., Hayashi, Y., Petralia, R. S., Zaman, S. H., Wenthold, R. J., Svoboda, K. and Malinow, R., 1999: Rapid spine delivery and redistribution of AMPA receptors after synaptic NMDA receptor activation, Science 284, 1811-1816.
Sholl, D. A., 1955: The surface area of cortical neurons. J. Anat. (London) 89, 571-572.
Silberberg, G., Gupta, A. and Markram, H., 2002: Stereotypy in neocortical microcircuits, Trends Neurosci. 25, 227-230.
Shu, T., Valentino, K. M., Seaman, C., Cooper, H.M. and Richards, L. J., 2000: Expression of the netrin-1 receptor, deleted in colorectal cancer (DCC), is largely confined to projecting neurons in the developing forebrain, J. Comp. Neurol. 416, 201-212.
Smetters, D. K., Hahm, J. and Sur, M., 1994: N-methyl-D-aspartate receptor antagonist does not prevent eyespecific segregation in the ferret retinogeniculate pathway, Brain Res. 658, 168-178.
Sretavan, D. W. and Shatz, C., 1986a: Prenatal development of retinal ganglion cell axons: Segregation into eye-specific geniculate layers from the intermixed state, J. Neurosci. 6, 234-251.
Sretavan, D. W. and Shatz, C. J., 1986b: Prenatal development of cat retinogeniculate axon arbors in the absence of binocular interactions, J Neurosci. 6, 990-1003
Star, E. N., Kwiatkowski, D. J. and Murthy, V. N., 2002: Rapid turnover of actin in dendritic spines and its regulation by activity, Nat. Neurosci. 5, 239-246.
Stellwagen, D. and Shatz, C. J., 2002: An instructive role for retinal waves in the development of retinogeniculate connectivity, Neuron 33, 357-367.
Steward, O. and Levy, W. B., 1982: Preferential localization of polyribosomes under the base of dendritic spines in granule cells of the dentate gyrus, J. Neurosci. 2, 284-291.
Steward, O., Wallace, C. S., Lyford, G. L. and Worley, P. F. 1998: Synaptic activation causes the mRNA for the IEG Arc to localize selectively near activated postsynaptic sites on dendrites. Neuron 21, 741-751.
Stryker, M. P. and Harris, W. A., 1986: Binocular impulse blockade prevents the formation of ocular dominance columns in cat visual cortex. J. Neurosci., 6, 2117-2133.
Sur, M. and Leamey, C. A., 2001: Development and plasticity of cortical areas and networks. Nat. Rev. Neurosci. 2, 251-262.
Sutton, J. K. and Brunso-Bechtold, J. K., 1993: Dendritic development in the dorsal lateral geniculate nucleus of ferrets in the postnatal absence of retinal input: A Golgi study, J. Neurobiol. 24, 317-334.
Tessier-Lavigne M. and Goodman C. S., 1996: The molecular biology of axon guidance, Science 274, 1123-1133.
Threadgill, R., Bobb, K. and Ghosh, A., 1997: Regulation of dendritic growth and remodeling by Rho, Rac and Cdc42, Neuron 19, 625-634.
Togashi, H., Abe, K., Mizoguchi, A., Takaoka, K., Chisaka, O. and Takeichi, M., 2002: Cadherin regulates dendritic spines morphogenesis, Neuron 45, 1-3.
Torre, E. R. and Steward, O., 1992: Demonstration of local protein synthesis within dendrites using a new culture system that permits the isolation of living axons and dendrites from their cell bodies, J. Neurosci. 12, 762-772.
Voyvodic, J. T., 1987: Development and regulation of dendrites in the rat superior cervical ganglion, J. Neurosci. 7, 904-912.
Weeks, A. C., Ivanco, T. L., Leboutillier, J. C., Racine, R. J. and Petit, T. L., 2001: Sequential changes in the synaptic structural profile following long-term potentiation in the rat dentate gyrus: III. Long-term maintenance phase. Synapse 40, 74-84.
Wiesel, T. N. and Hubel, D. H., 1965: Comparison of the effects of unilateral and bilateral eye closure on cortical unit responses in kittens, J. Neurophysiol. 28, 1029-1040.
Williams, C. V., Davenport, R. W., Dou, P. and Kater, S. B., 1995: Developmental regulation of plasticity along neurite shafts, J. Neurobiol. 27, 127-140.
Whitford, K. L., Dijkhuizen, P., Polleux, F., and Ghosh, A., 2002: Molecular control of cortical dendrite development, Annu. Rev. Neurosci. 25, 127-149.
Wong, R., Yamawaki, R. and Shatz, C., 1992: Synaptic contacts and the transient dendritic spines of developing retinal ganglion cells, Eur. J. Neurosci. 4, 1387-1397.
Wu, G.-Y., Zou, D. G., Rajan, I. and Cline, H., 1999: Dendritic dynamics in vivo change during neuronal maturation, J. Neurosci. 19, 4472-4483.
Wu, H. H., Williams, C. V. and Mcloon, S. C., 1994: Involvement of nitric oxide in the elimination of a transient retinotectal projection in development, Science 265, 1593-1596.
Xia, Z., Dudek, H., Miranti, C. K., Greenberg, M. E., 1996: Calcium influx viaNMDA receptor induces immediate early gene transcription by a MAP Kinase/ERK-dependent mechanism, J. Neurosci. 16, 5425-5436.
Yao, W. D., Rusch, J., Poo, M.-M., Wu, C. F., 2000: Spontaneous acetylcholine secretion from developing growth cones of Drosophila central neurons in culture: Effects of cAMP-pathway mutations, J. Neurosci. 20, 2626-2637.
Yawo, H., 1987: Changes in the dendritic geometry of mouse superior cervical ganglion cells following postganglionic axotomy, J. Neurosci. 7, 3703-3711.
Ziv, N. E. and, Smith, S., 1996: Evidence for a role of dendritic filopodia in synaptogenesis and spine formation, Neuron 17, 91-102.
Zou, D. J. and Cline, H. T., 1999. Postsynaptic calcium/calmodulin Kinase II is required to limit elaboration of presynaptic and postsynaptic arbors, J. Neurosci. 19, 8909-8918.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Rocha, M., Furtado, D.A., Menezes, J.R.L. et al. Form and Function: A Neuronal Dialog. Brain and Mind 4, 3–25 (2003). https://doi.org/10.1023/A:1024156030989
Issue Date:
DOI: https://doi.org/10.1023/A:1024156030989